All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Results from the phase III AALL1331 trial: Blinatumomab in low-risk children and AYA patients with B-ALL in first relapse
Do you know... In patients with low-risk B-ALL, which of the following sites of first relapse did blinatumomab significantly improve disease-free and overall survival rates for?
Despite significant improvements in survival rates for children with newly diagnosed acute lymphoblastic leukemia (ALL), 10–15% still experience relapse.1 The 5-year overall survival (OS) rates for children and young adults with B-cell ALL (B-ALL) at first relapse are poor, ranging from 35–50%.1
Time from diagnosis, site of relapse, and minimal residual disease (MRD) status after 4 weeks of re-induction chemotherapy are key predictors of outcomes at first relapse. Patients with bone marrow (BM) relapse with or without an extramedullary (EM) relapse ≥36 months, or isolated EM relapse ≥18 months from diagnosis with low MRD (0.1%) at the end of re-induction chemotherapy, have historically been classified as low-risk (LR) due to their favorable prognoses.
Blinatumomab is a U.S. Food and Drug Administration (FDA) approved bispecific T-cell engager for the treatment of adults and children with relapsed/refractory B-ALL and MRD-positive B-ALL. Previous results from the AALL1331 Children’s Oncology Group study (NCT02101853) demonstrated improved OS and reduced toxicities with blinatumomab in high- and intermediate-risk patients at first B-ALL relapse.1
The ALL Hub previously reported the efficacy and safety results for LR patients treated with blinatumomab at first-relapse of B-ALL. Here, we summarize the full trial results, published by Hogan et al. in The Journal of Clinical Oncology.1
Study design
AALL1331 is a randomized phase III trial which included patients aged 1–30 years with B-ALL at first relapse. Patients who completed end re-induction risk assessment and were classified as LR were included. Relapses were defined as follows:
- A bone marrow (BM) relapse is defined as >25% BM blasts after remission, and isolated BM relapse was defined as >25% BM blasts without the involvement of the central nervous system (CNS) and/or testicles.
- CNS relapse is defined as positive cytomorphology and a white blood cell count of ≥5/ul or clinical signs of CNS leukemia.
- Isolated extramedullary relapse represents a CNS or testicular relapse with <5% BM blasts.
- Combined relapse was classified as ≥5% BM blasts with CNS or testicular relapse.
The study design is shown in Figure 1. The primary endpoint was disease-free survival (DFS), defined as the time from randomization until relapse, second malignancy, or death. The secondary endpoint was OS, defined as the time from randomization until death from any cause.
Figure 1. Study design*
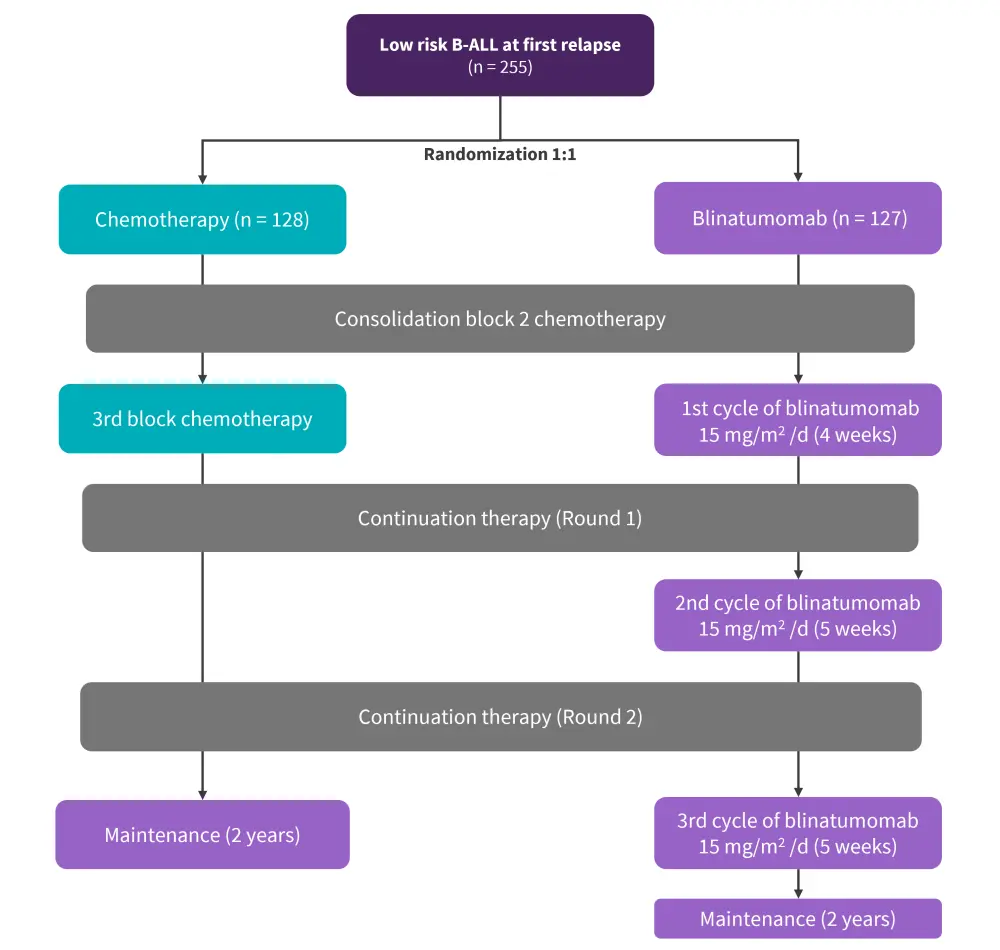
B-ALL, B-cell acute lymphoblastic leukemia
*Data from Hogan, et al.1
Results
Of the 255 patients with LR B-ALL at first relapse, 128 received chemotherapy and 127 received blinatumomab + chemotherapy. The baseline characteristics were similar between the two treatment arms, as summarized in Table 1.
Table 1. Baseline characteristics*
|
BM, bone marrow; EM, extramedullary; IBM, isolated bone marrow; IQR, interquartile range. |
||
|
Characteristic, % (unless otherwise stated) |
Blinatumomab |
Chemotherapy |
|---|---|---|
|
Age at enrolment, years |
||
|
Median (IQR) |
11 (7−15) |
10 (7−15) |
|
1–12 |
66.1 |
66.4 |
|
13–20 |
29.1 |
30.5 |
|
21–26 |
4.7 |
3.1 |
|
Age at initial diagnosis |
||
|
Median (IQR) |
6 (3−9) |
5 (2−9) |
|
<1–9 |
78 |
75.8 |
|
10–17 |
22 |
21.9 |
|
18–23 |
― |
2.3 |
|
Sex |
|
|
|
Male |
59.8 |
59.4 |
|
Female |
40.2 |
40.6 |
|
Site of relapse |
|
|
|
IBM |
55.1 |
56.3 |
|
BM plus EM |
13.4 |
11.7 |
|
Isolated EM |
31.5 |
32 |
|
Cytogenetic group† |
|
|
|
Favorable |
32.5 |
35.6 |
|
Unfavorable |
4.2 |
1.9 |
|
Other |
63.3 |
62.5 |
- At a median follow-up of 3.5 years, 97 DFS events occurred, 42 in the blinatumomab arm and 55 in the chemotherapy arm.
- The 4-year DFS for the overall LR cohort was 55.2% and the 4-year OS was 84.9%.
- Overall, the 4-year DFS and OS rates were higher in the blinatumomab arm compared with the chemotherapy arm; however, this difference was not significant.
- There were significant differences in the DFS and OS rates between the two arms among patients with BM ± EM relapse and isolated BM relapse, as shown in Figure 2.
- For isolated CNS relapses, the 4-year DFS rates were 24.7% vs 24% for blinatumomab vs chemotherapy, respectively.
- The 4-year OS rates were 75% and 60.3%, respectively.
- For isolated testicular relapses, the 4-year DFS and OS rates were 77.8% and 88.9% for blinatumomab vs 100% each for chemotherapy.
- The median time from randomization until cranial radiation therapy was 10 months for all assigned patients vs 12 months for both treatment arms.
- The 3-year OS after the second relapse was 75.4% and 60.5% for blinatumomab vs chemotherapy, respectively.
Figure 2. 4-year A OS and B DFS rates in blinatumomab vs chemotherapy arm according to the site of relapse*
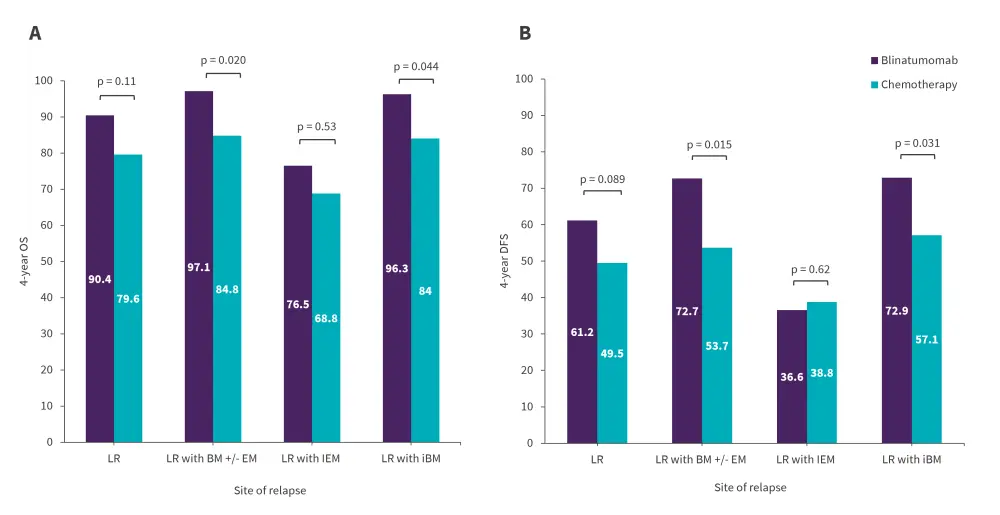
BM, bone marrow; EM, extramedullary site; iBM, isolated bone marrow relapse; IEM, isolated extramedullary relapse; LR, low risk.
*Data from Hogan, et al.1
- In patients with isolated extramedullary relapse, the site of first relapse (testes vs CNS, p = 0.017) and the time from diagnosis to first relapse (>36 vs 18–36 months; p = 0.017) were the only significant predictors of DFS.
- Multivariate analyses showed that younger patients, blinatumomab vs chemotherapy, later relapses, and undetectable MRD in the BM ± EM relapsed cohort were significantly associated with DFS (Figure 3).
Figure 3. Cox aggression model of DFS rates across various subgroups with iBM or BM ± EM relapse*†
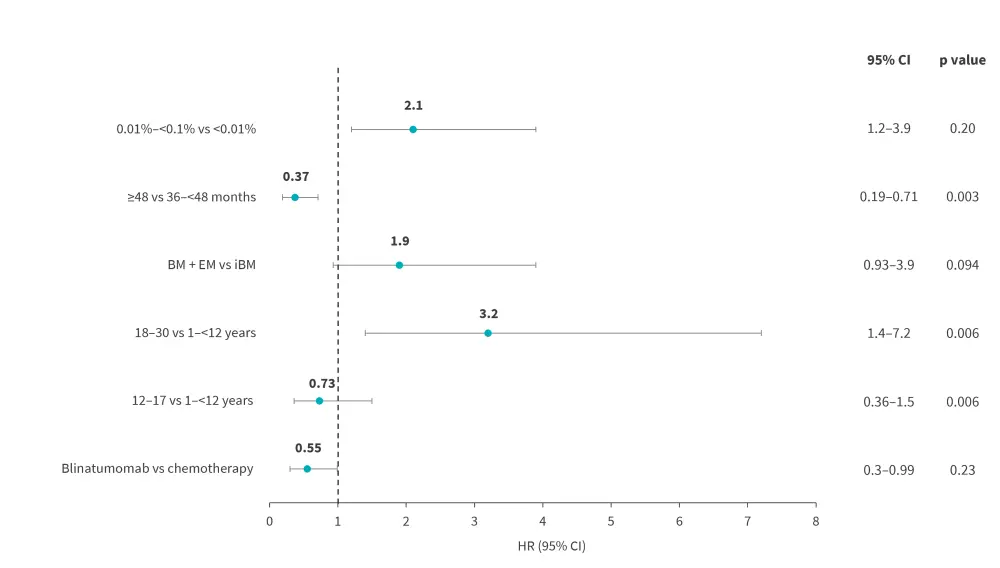
BM, bone marrow; EM, extramedullary site; DFS, disease free survival; iBM, isolated bone marrow;
*Data from Hogan, et al.1
†p value for comparison of blinatumomab arm versus chemotherapy arm was one-sided, whereas p value for comparison of patient/disease characteristics was two-sided.
Safety analysis
- Grade 3–5 adverse events (AEs) of special interest were significantly lower in the first cycle of blinatumomab arm vs 3rd block of chemotherapy (Figure 4).
Figure 4. AEs of interest in 3rd block chemotherapy vs 1st blinatumomab arm *
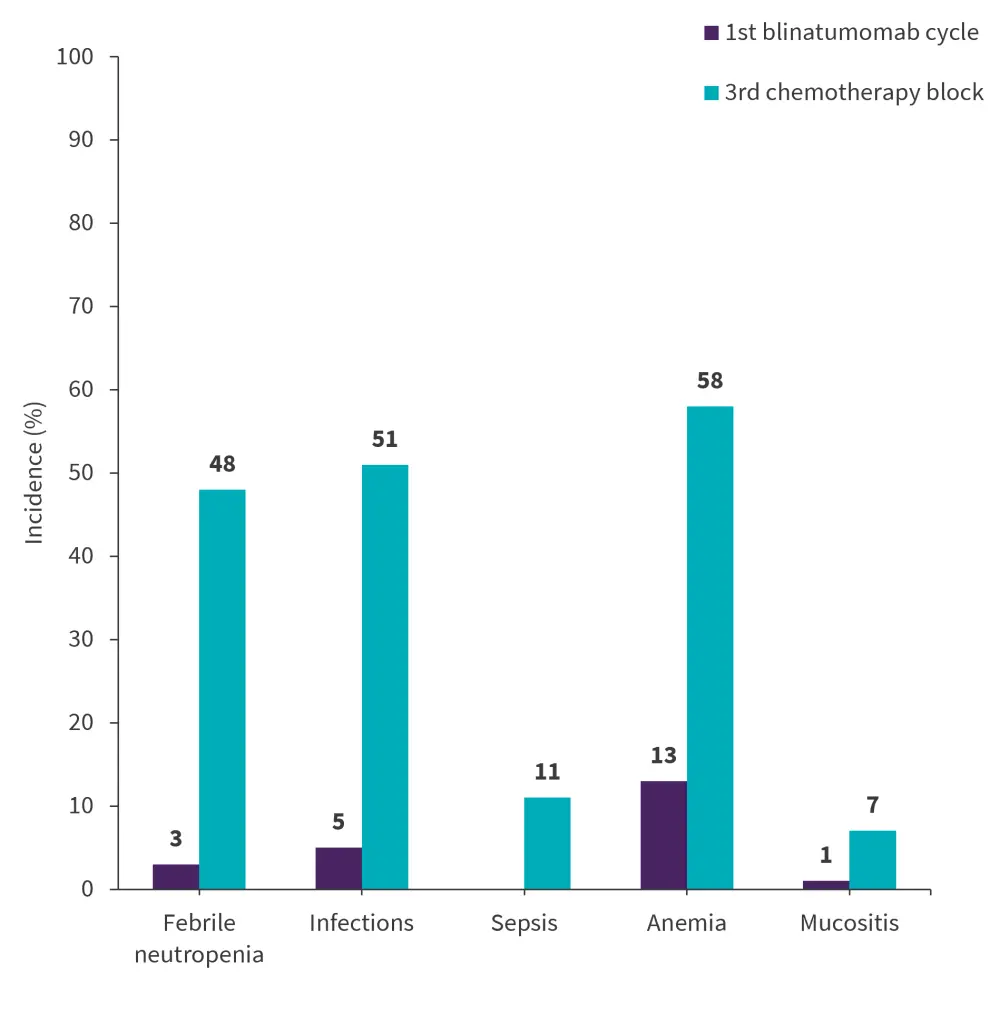
AEs, adverse events.
*Data from Hogan, et al.1
- In patients included in the safety analysis, any-grade AEs occurred in 89% and 97% of patients in the chemotherapy block 3 arm versus blinatumomab arm combined after Cycle 1, 2, and 3.
- Grade ≥3 AEs occurred in 87% and 86%, respectively.
- Selected any-grade blinatumomab-related AEs occuring after combined Cycle 1, 2, and 3 included cytokine release syndrome in 15%, seizures in 6%, and encephalopathy in 29% of patients.
- There were four Grade 5 AEs of death reported, three in the chemotherapy and one in the blinatumomab arm.
Conclusion
This randomized study showed that overall, there was no statistically significant difference in DFS or OS between the blinatumomab and standard chemotherapy arms for children, adolescent, and young adult patients with low-risk first relapse of B-ALL. However, blinatumomab significantly improved DFS and OS for patients with BM with or without EM relapse and those with isolated BM relapse, thus establishing a new standard of care for these populations. Notably, blinatumomab was well-tolerated overall, with significantly lower severe hematologic and infectious toxicity compared with patients treated with standard chemotherapy. Furthermore, patients with isolated CNS relapse had poor outcomes with or without blinatumomab; therefore, future studies should investigate new treatment strategies for these patients.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



