All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Sequential treatment with blinatumomab and inotuzumab in patients with relapsed B-ALL
Patients with relapsed or refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) have extremely poor outcomes. Monoclonal antibody (mAb)-based treatments such as blinatumomab and inotuzumab targeting CD19, CD20, and CD22 have changed the treatment landscape for patients with B-ALL. In comparison to conventional chemotherapy, both blinatumomab and inotuzumab show superior antileukemic activity and are favored for salvage treatment, including use as a bridging treatment for allogeneic hematopoietic stem cell transplantation (allo-HSCT) in patients with R/R B-ALL.
There is limited evidence on the efficacy of blinatumomab and inotuzumab in patients resistant to both mAbs as well as on alternating them in patients who relapse after the use of blinatumomab or inotuzumab. The clinical benefit and safety of allo-HSCT after both blinatumomab and inotuzumab also remains uncertain. The increased use of blinatumomab and inotuzumab in clinical practice warrants an understanding of the efficacy and safety of these two mAbs when used sequentially. Below, we present the key findings from a recently published study by Wudhikarn, et al.,1 in Blood Advances reporting the outcomes of patients with R/R B-ALL previously treated with blinatumomab and inotuzumab.
Study design
This was a cohort study comprising of patients aged ≥15 years diagnosed with R/R B-ALL who had received salvage therapy for morphologic or minimal residual disease (MRD) with both blinatumomab and inotuzumab between January 2012 and December 2019 at the Memorial Sloan Kettering Cancer Center, USA.
Results
Baseline characteristics
A total of 29 patients were included, of which 25 received blinatumomab as first mAb (mAb1) and inotuzumab as second mAb (mAb2) while the remaining four received inotuzumab as mAb1 and blinatumomab as mAb2. Complete remission (CR) was achieved by 16 and 19 patients after mAb1 and mAb2, respectively. The baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics*
|
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; B-ALL, B-cell acute lymphoblastic leukemia; CR, complete response; IQR, interquartile range; mAb, monoclonal antibody; Ph+, Philadelphia chromosome positive; MRD, minimal residual disease. *Wudhikarn, et al.1 †Based on UKALLXII/ECOG2993. |
|||
|
Characteristics, % (unless otherwise stated) |
All patients |
Blinatumomab |
Inotuzumab |
|---|---|---|---|
|
Median age (IQR), years |
45.3 (25.1–62.6) |
43.6 (24.4–60.7) |
61.2 (54.6–70.6) |
|
Sex, male |
58.6 |
52.0 |
100.0 |
|
Cytogenetics† |
|||
|
High risk |
41.4 |
40.0 |
50.0 |
|
Standard risk |
48.3 |
48.0 |
50.0 |
|
Ph+ B-ALL |
13.8 |
16.0 |
0.0 |
|
Presence of extramedullary disease |
27.6 |
24.0 |
50.0 |
|
CR to first line induction therapy |
|||
|
Relapse after achieving CR |
82.8 |
88.0 |
50.0 |
|
Refractory |
17.2 |
12.0 |
50.0 |
|
Median no. of prior treatment lines before first mAb (range), lines |
1 (1–5) |
1 (1–5) |
1 (1–2) |
|
Prior allo-HSCT before mAb1 |
10.3 |
12.0 |
0.0 |
|
Prior CD19 CAR T-cell therapy before mAb1 |
10.3 |
12.0 |
0.0 |
|
MRD at time of mAb1 |
24.1 |
28.0 |
0.0 |
|
Median time from mAb1 to mAb2 (IQR), days |
99 (35–240) |
99 (35–240) |
116 (55–221) |
|
Interim treatment between 2 mAbs |
48.3 |
52.0 |
25.0 |
|
Median number of blinatumomab (range), cycles |
1 (1–6) |
1 (1–6) |
2 (1–5) |
|
Median number of inotuzumab (range), cycles |
2 (1–5) |
2 (1–5) |
2 (1–2) |
|
Allo-HSCT after mAb2 |
41.4 |
44.0 |
25.0 |
Blinatumomab – mAb1
Of the 25 patients who received blinatumomab mAb1, 52% achieved CR or CR with incomplete recovery (CRi) including MRD negative (MRD−) CR in 32% of patients. Overall, 44% of patients progressed with CD19 negative/dim (CD19−/dim) disease, whereas the remaining 56% had persistent CD19 expression (CD+) (Figure 1). Cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome of all grades were observed in 52% and 28% of patients, respectively. Six patients underwent allo-HSCT after blinatumomab mAb1 however, all subsequently relapsed before receiving inotuzumab mAb2.
CR was achieved in 17 patients (68%) after inotuzumab mAb2, including MRD− CR in 48% of patients. The CR rates between patients with CD19−/dim and CD19+ relapse post-blinatumomab were similar (8/11 vs 9/14 patients, respectively), and there was no significant difference between CR rates for patients who received non-mAb-based interim therapy after blinatumomab mAb1 (n = 13) and those who proceeded straight to inotuzumab mAb2 (69.2% vs 66.7%; p = 0.89).
Of the 17 patients who achieved CR after inotuzumab mAb2, ten underwent consolidative allo-HSCT. Fourteen patients developed hepatotoxicity and four patients met the diagnostic criteria of hepatic sinusoidal obstructive syndrome post-allo-HSCT.
Of the 25 patients treated with blinatumomab mAb1/inotuzumab mAb2 19 died, including 10 with CD19−/dim disease and 9 with CD19+ disease. The causes of death included progressive disease and treatment-related mortality in 14 and 5 patients, respectively.
Figure 1. Consort diagram*
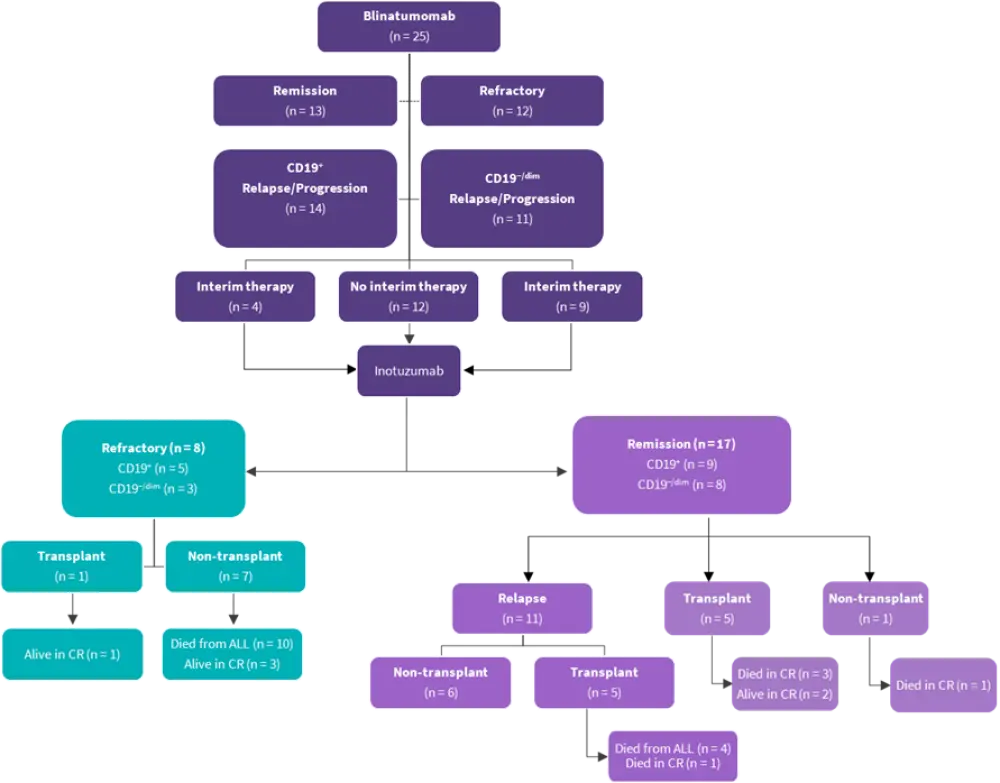
ALL, acute lymphoblastic leukemia; CR, complete remission; dim, dim expression.
*Wudhikarn, et al.1
Inotuzumab – mAb1
Of the four patients who received inotuzumab mAb1, three achieved CR and one had persistent disease (Figure 2). Amongst the three patients achieving CR, one received blinatumomab mAb2 as a bridge to allo-HSCT while the remaining two received it as consolidation therapy. However, all three patients subsequently relapsed.
One patient developed Grade 1 cytokine release syndrome and Grade 1 immune effector cell-associated neurotoxicity syndrome after blinatumomab mAb2. In total two patients died, one from progressive disease and one from treatment-related toxicity. One patient remains alive in CR after inotuzumab re-treatment, and one remains alive with persistent disease after receiving non-mAb-based salvage treatments.
Figure 2. Consort diagram – inotuzumab mAb1*
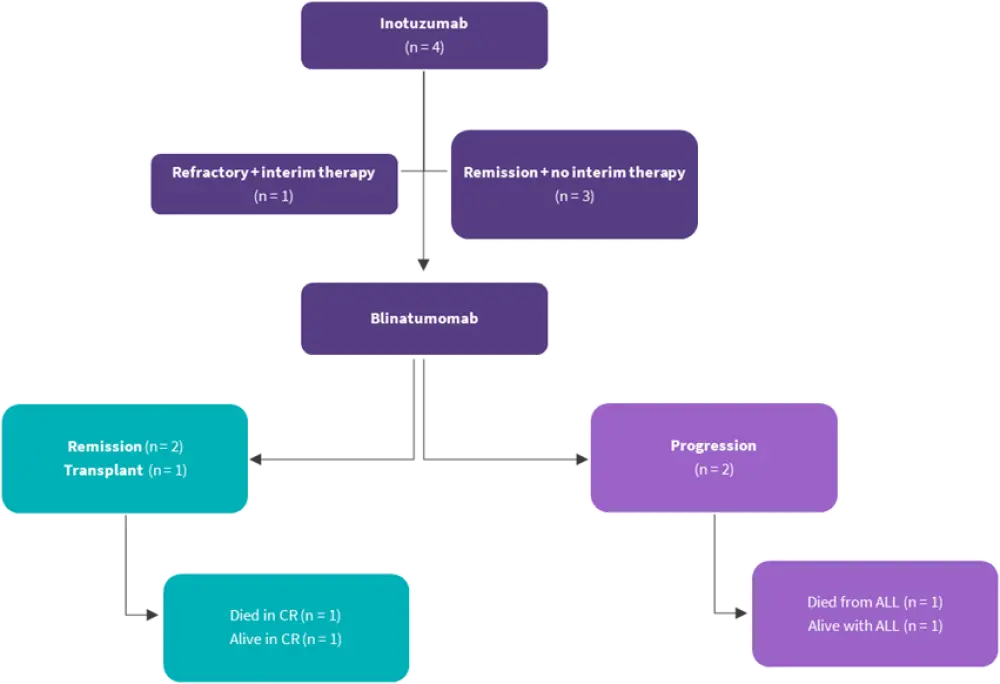
ALL, acute lymphoblastic leukemia; CR, complete remission
*Wudhikarn, et al.1
Survival outcomes
Survival analysis was focused on the patients who received blinatumomab mAb1/inotuzumab mAb2 since most patients received this regimen. The 1-year event-free survival after blinatumomab mAb1 was 16.0% (95% confidence interval [CI], 6.5–35.2) which was similar between CD19−/dim and CD19+ relapse (9.1% vs 21.4%; p = 0.48). There was no difference in 1-year overall survival between the following patient groups:
- CD19−/dim vs CD19+ relapse post-blinatumomab mAb1 (45.5% vs 35.7%; p = 0.73)
- CR vs no CR after blinatumomab mAb1 (38.5% vs 41.7%; p = 0.61)
- Received vs did not receive interim non-mAb-based salvage therapy for relapse after blinatumomab mAb1 (46.2% vs 33.3%; p = 0.82)
- Responded vs did not respond to inotuzumab mAb2 (41.2% vs 37.5%; p = 0.71)
Overall, 12 patients underwent allo-HSCT after mAb2, 10 post-inotuzumab mAb2, one post-blinatumomab mAb2, and one after CD19 chimeric antigen receptor (CAR) T-cell therapy post-inotuzumab mAb2. Acute GvHD was observed in seven patients, including Grade ≥3 in three patients. The 1-year event-free survival and overall survival after allo-HSCT were 25% and 31.2%, respectively.
Conclusion
This cohort study demonstrated that although patients with B-ALL who relapsed after blinatumomab could be rescued by inotuzumab, and allo-HSCT may offer possible long-term remission in a subgroup of patients, the incidence of treatment-related mortality and relapse remained high. The findings were limited by the small sample size, selection bias, and retrospective nature of the study. In addition, heterogeneity of salvage therapy, concomitant treatments, and allo-HSCT platforms may have been potential confounding factors. Further research exploring approaches to selection criteria for transplantation and novel conditioning regimens with optimal safety profiles are needed to improve allo-HSCT outcomes. Also, the role of CAR T-cell therapy after blinatumomab and inotuzumab as consolidation therapy needs to be examined.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

