All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Survival outcomes in patients with Ph+ ALL following failure with a ponatinib-based regimen
The standard of care for the frontline treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) is chemotherapy or blinatumomab plus BCR-ABL1 tyrosine kinase inhibitor (TKI). Ponatinib-based regimens have achieved promising outcomes in Ph+ ALL; however, some patients still experience relapse. The outcomes and characteristics of patients who relapse following a frontline ponatinib-based regimen is not known.1
Here, we summarize a retrospective study published by Short et al.1 in the American Journal of Hematology investigating the outcomes of patients with Ph + ALL following the failure of a ponatinib-based regimen.
Study methods1
- This retrospective study included patients with Ph+ ALL who received a frontline ponatinib-based regimen and subsequently experienced a hematologic and/or extramedullary relapse.
- Key endpoints include relapse-free survival (RFS) and overall survival (OS).
Key findings1
- Between March 2012 and September 2023, a total of 22 patients were included in the analysis, 73% of whom were ponatinib-resistant and 27% were ponatinib-intolerant.
- The median time from diagnosis to relapse for ponatinib-resistant and ponatinib-intolerant patients was 24.1 months and 31.4 months, respectively.
- The type of relapses that occurred included:
- bone marrow only (54%);
- central nervous system (CNS) only (27%);
- bone marrow with CNS and/or non-CNS extramedullary involvement (14%); and
- non-CNS extramedullary only (5%).
- Overall, 91% of patients received ≥1 subsequent therapy following ponatinib failure.
- All patients received a TKI-based regimen in first salvage (ponatinib [40%]; dasatinib [35%]; bosutinib [20%]; and olverembatinib [5%]).
- Alongside TKIs, patients received either chemotherapy (65%), blinatumomab (30%), inotuzumab ozogamicin (20%), and CD19-directed chimeric antigen receptor T-cell therapy (35%).
- Following the first salvage therapy, 90% of patients achieved a second remission.
- All patients who received blinatumomab, inotuzumab ozogamicin, and CAR T-cell therapies achieved a complete remission or complete remission with incomplete count recovery.
- At a median follow-up of 18.6 months, the median RFS and OS for the total cohort was 14.7 months and 22.6 months, respectively.
- The 1-year and 2-year RFS was 55% and 37%, respectively.
- The median OS for patients who experienced relapse in the bone marrow and extramedullary-only was 15.7 months and not reached, respectively.
- OS rates for the total cohort and by relapse are reported in Figure 1.
Figure 1. 1-and 2-year OS in the total cohort and by type of relapse*
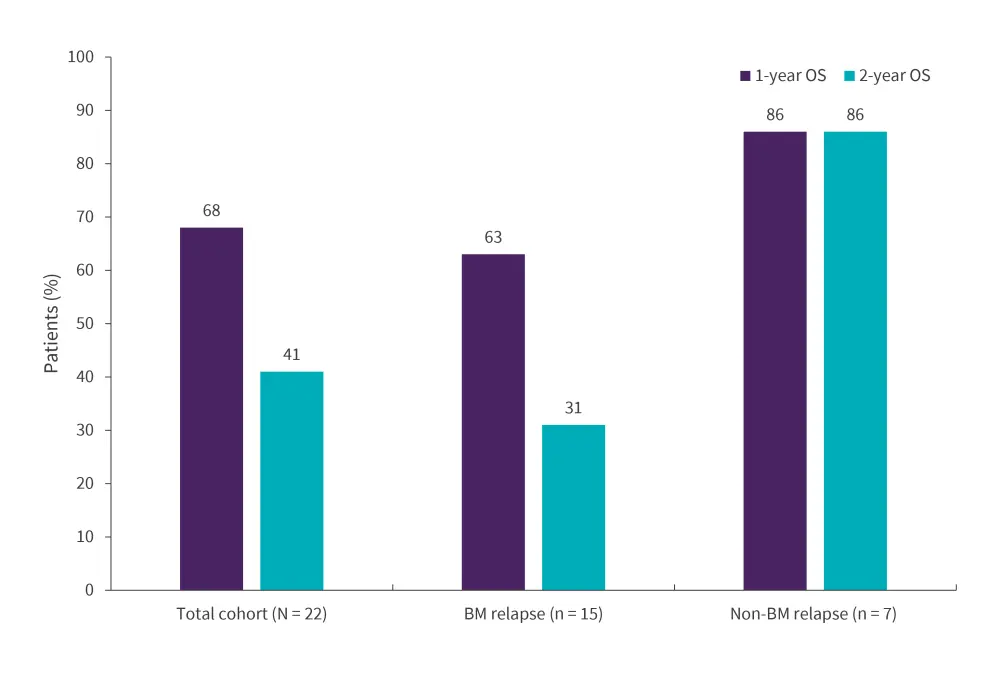
BM, bone marrow; OS, overall survival.
*Data from Short, et al.1
| Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

