All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
The addition of blinatumomab to the Interfant 06 study protocol for infants with newly diagnosed ALL
Infants (<1 year old) with acute lymphoblastic leukemia (ALL) have worse survival outcomes than older children, which is further pronounced with the presence of KMT2A rearrangement (KMT2A-r). KMT2A-r is common in infant patients (75%) and produces high rates of early relapse which is associated with reduced survival (20%). Improvements to frontline treatment are therefore of urgent need. One potential strategy is the use of blinatumomab, an anti-CD19 bispecific T-cell engager, which has previously demonstrated promising efficacy and safety in both older children and adult patients with ALL.1
At the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition, Inge Van Der Sluis presented a phase II study investigating the safety and efficacy of blinatumomab as an addition to the Interfant-06 study treatment protocol in infants with newly diagnosed KMT2A-r ALL.1 We summarize key findings below.
Study design
This was a phase II prospective, open-label, non-randomized, multicenter pilot study. The study design, including primary and secondary endpoints, is summarized in Figure 1.
Figure 1. Study Design*
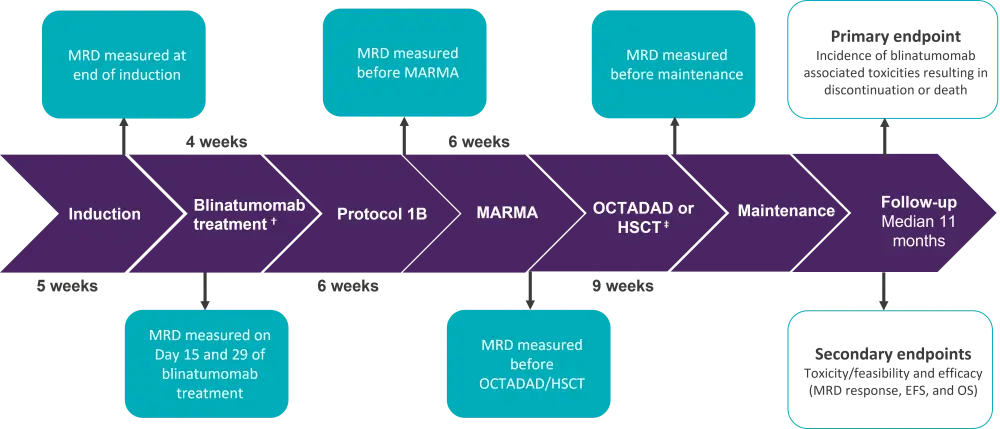
EFS, event-free survival; HSCT, hematopoietic stem cell transplant; MRD, minimal residual disease; OS, overall survival.
*Adapted from Van Der Sluis, et al.1
ⴕ15 ug/m2/day, 28-day continuous infusion.
‡Medium risk patients with MRD levels >0.05% before OCTADAD and all high-risk patients in complete remission were eligible for HSCT.
Eligibility criteria for this study included:
- treatment according to Interfant-06 backbone
- newly diagnosed, CD19 positive, B-precursor ALL
- <1 year of age
- KMT2Ar-ALL
- M1 or M2 bone marrow after induction
- no clinically relevant CNS pathology
- no evidence of CNS involvement at the end of induction
Results
Patient characteristics for the entire cohort (N = 30) are summarized in Table 1.
Table 1. Patient characteristics*
|
CNS, central nervous system; CR, complete remission; KMT2Ar, KMT2A rearrangement; MRD, minimal residual disease. |
|
|
Characteristic, % |
N = 30 |
|---|---|
|
Age at diagnosis |
|
|
<6 months |
63 |
|
≥6 months |
37 |
|
Female |
60 |
|
Medium risk |
70 |
|
High riskⴕ |
30 |
|
CR at end of induction |
90 |
|
CNS status at diagnosis |
|
|
CNS1 |
30 |
|
CNS2 |
43 |
|
CNS3 |
10 |
|
Not evaluable/unknown |
17 |
|
KMT2Ar status |
|
|
t(4;11) |
50 |
|
t(9;11) |
10 |
|
t(11;19) |
20 |
|
Other/partner not known |
20 |
|
MRD < 0.05% |
60 |
Safety
All patients completed a 4-week course of blinatumomab, and only one patient interrupted treatment for two days due to hypertensive crisis. Serious adverse events (AEs) during blinatumomab treatment were reported in ten patients, including infections Grade 3–4 (n = 4), fever (n = 4), vomiting (n = 1), and hypertensive crisis (n = 1).
A total of 80 AEs were reported, and the most common Grade ≥3 AEs included febrile neutropenia (n = 2), anemia (n = 10), and elevated gamma-glutamyl transferase (n = 2).
MRD response
- The rate of minimal residual disease (MRD) negativity/MRD <5 × 104 was 93% the end of blinatumomab treatment.
- MRD negativity/MRD <5 × 104 before OCTADAD/HSCT was 100%, significantly higher when compared to historical controls receiving IF06 study treatment (82%; p = 0.02).
- Patients who were classified with medium risk had higher rates of MRD negativity at the end of blinatumomab treatment compared with high-risk patients (62% vs 22%; p = 0.0457)
- Likewise, those with low MRD (<5 × 104) at the end of induction showed significantly higher MRD negativity at the end of blinatumomab treatment (78% vs 18%; p = 0.0022).
- No patients with medium risk were given HSCT based on high MRD before OCTADAD, compared with 20% in the interfant-06 trial.
- However, two medium risk patients received HSCT based on high MRD at the end of induction.
Relapse and death
- One patient died due to toxicity which was not related to blinatumomab.
- Three relapses were reported, including two patients who were medium risk and are in second complete remission following HSCT. One patient with high-risk disease who relapsed died.
- Four patients had a CNS or combined bone marrow-CNS relapse, all of which were CD19 positive.
EFS and OS
- After a median follow-up of 16.3 months, the 1-year event-free survival (EFS) rate was 90% and 1-year overall survival (OS) rate was 93.1%.
- In comparison, historical controls treated with IF06 had a 1-year EFS of 54.8% and OS rate of 69.8%.
Conclusion
This study demonstrated that the addition of blinatumomab to the interfant-06 study protocol was well tolerated in infants with ALL. MRD response was high, relapse rates were low, and EFS was greater than historical controls. Despite a short follow-up, Van Der Sluis highlighted that most events in the blinatumomab treatment group occurred in the first year, which is encouraging for long-term outcomes. Relapse will continue to be monitored in the future, and a new interfant-06 protocol will include blinatumomab.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

