All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Tisagenlecleucel therapy in infants and children ≤3 years of age with relapsed/refractory B-cell ALL
Introduction
A majority of pediatric patients with high-risk B-cell precursor acute lymphoblastic leukemia (ALL) have infant ALL, a rare disease subtype that carries a poor prognosis and suboptimal outcomes with standard treatment modalities such as chemotherapy and hematopoietic stem-cell transplantation (HSCT). Previous cooperative studies have not shown significant improvement. The Interfant-99 cohort demonstrated a 6-year event-free survival (EFS) of 46.5% and 6-year overall survival (OS) of 53.8% and the Interfant-06 cohort showed a 6-year EFS of 46.1% with a 6-year OS of 58.2%. Furthermore, the Interfant-06 protocol revealed KMT2A-rearranged leukemia as being a particularly high-risk form of the disease.1
The phase II ELIANA trial (NCT02435849), previously reported on the ALL Hub, demonstrated the long-term durable efficacy and safety of tisagenlecleucel (tisa-cel) in this difficult-to-treat patient population; however, children aged ≤3 years were excluded and the precise efficacy and safety of tisa-cel in this patient cohort is still unclear. Below, we provide a summary of an international, multicenter, retrospective cohort study on the role of tisa-cel treatment in infants and children aged ≤3 years with relapsed/refractory B-cell ALL, as published by Ghorashian et al.1 in The Lancet Haematology in 2022.
Study design
Overall, 38 patients were selected from 15 hospitals across ten European countries (Table 1). Eligible patients had relapsed/refractory B-cell precursor ALL and were aged <3 years at the time of screening for tisa-cel therapy. Eligibility was assessed based on the summary of product characteristics for tisa-cel, including factors such as refractory disease status, posttransplant relapse history, and occurrence of ≥2 B-ALL relapses.
OS, EFS, stringent EFS (SEFS), B-cell aplasia, and toxicity were assessed in all patients who received tisa-cel infusion. Minimal residual disease (MRD) was assessed by flow cytometry or quantitative polymerase chain reaction (qPCR), depending on local practice.
Data on key factors associated with CAR T-cell therapy (disease characteristics, leukapheresis product, bridging therapy, CAR T-cell dose, toxicities, disease response, and long-term outcome) was collected using a standardized data reporting method circulated to all participating centers.
Table 1. Baseline and clinical characteristics*
|
CNS, central nervous system; HSCT, hematopoietic stem cell transplantation; IQR, interquartile range; MRD, measurable residual disease. |
|
|
Characteristic, % (unless otherwise stated) |
Patients (N = 38) |
|---|---|
|
Median age at diagnosis, months |
5.2 |
|
Sex |
|
|
Male |
55 |
|
Female |
45 |
|
White blood cell count at diagnosis (IQR), × 109 cells/L |
375 (130–797) |
|
Presenting with CNS involvement |
47 |
|
Treated according to Interfant-06 protocol |
82 |
|
KMT2A arrangement |
76 |
|
Refractory to ≥1 previous treatment lines |
50 |
|
Prior HSCT |
66 |
|
Previous lines of therapy not including HSCT (IQR), n |
2 (2–3) |
|
Prior inotuzumab |
18 |
|
Prior blinatumomab |
37 |
|
Patients who received a tisagenlecleucel infusion (n = 35) |
|
|
Median age at infusion, months |
17 |
|
Bone marrow disease burden before |
|
|
Median (IQR) |
1.75 (0.2–31.0) |
|
MRD negative |
20 |
|
0–<1% |
14 |
|
1–<5% |
14 |
|
5–<10% |
6 |
|
10–<50% |
26 |
|
50–100% |
20 |
|
CNS disease before lymphodepletion |
3 |
Patients received a single intravenous infusion of tisa-cel, with follow-up conducted as per institutional policy on the delivery of chimeric antigen receptor (CAR) T-cell therapy (Figure 1).
Figure 1. Study design*
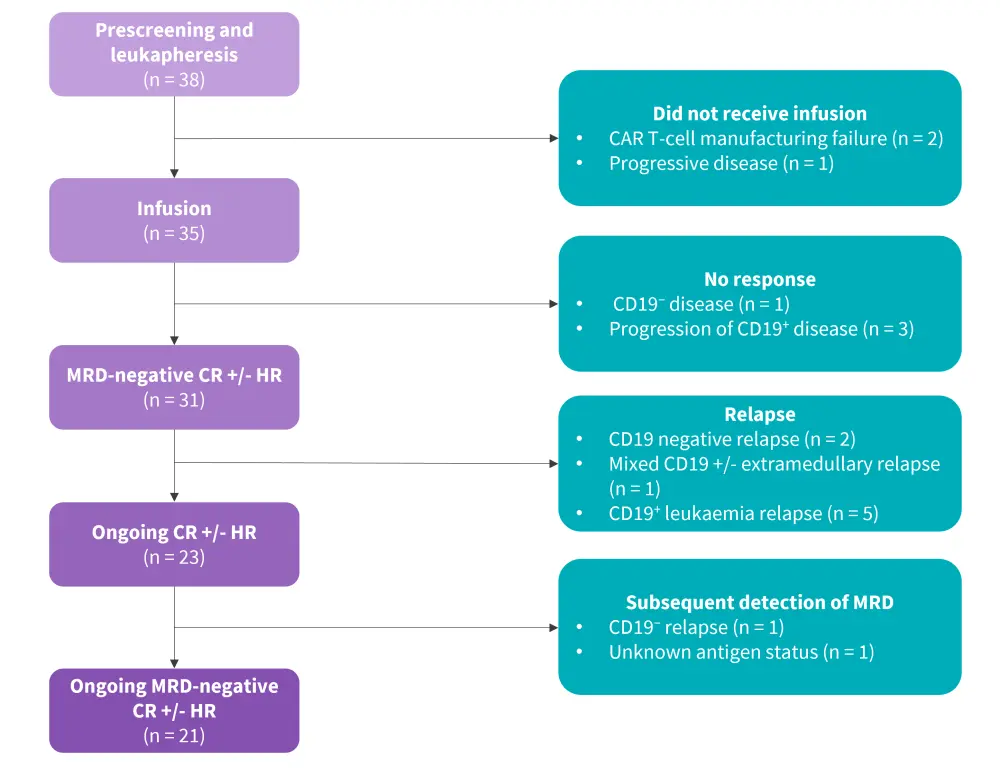
CAR, chimeric antigen receptor; CR, complete response; HR, hematological recovery; MRD, measurable residual disease.
*Adapted from Ghorashian, et al.1
Results1
Efficacy
- Of the 28 patients who received a tisa-cel infusion and were eligible for assessment, 86% had a complete response with or without hematological recovery; all responses were reached within 30 days and were associated with MRD negativity.
- Overall, seven patients received an infusion upon attaining MRD-negative bone marrow status after bridging therapy and without measurable extramedullary disease; all of these patients remained MRD-negative 30 days post-infusion.
- All four patients in which therapy did not yield a response subsequently died due to disease progression within 3 months of the infusion.
- After a median follow-up time of 14 months, the 6- and 12-month OS was 88% vs 84%, EFS was 75% vs 69%, and SEFS was 63% vs 41%, respectively.
- Among patients who received tisa-cel and had no MRD (n = 7), EFS was 100% and SEFS was 83% and 67% at 6 and 12 months, respectively.
- One patient experienced the emergence of MRD and received further therapy at 6 months and two patients had B-cell recovery before 6 months, leading to further treatment in one patient.
- Two patients had late disease relapses (>1 year after the infusion) and went on to receive further therapy.
- Survival analysis showed no significant association between pretreatment risk factors and outcome measures.
- Relapse risk was not significantly increased in patients with KMT2-rearranged leukemia.
- Among patients who reached complete response, 26% subsequently relapsed during the follow-up period.
- The cumulative incidence of relapse for patients who received a tisa-cel infusion was 19% (95% confidence interval [CI], 9–37) at 6 months and 25% (95% CI, 12–44) at 12 months.
- Among patients who received an infusion, 43% required further treatment during follow-up and 17% received allogeneic HSCT.
Figure 2. Efficacy results*
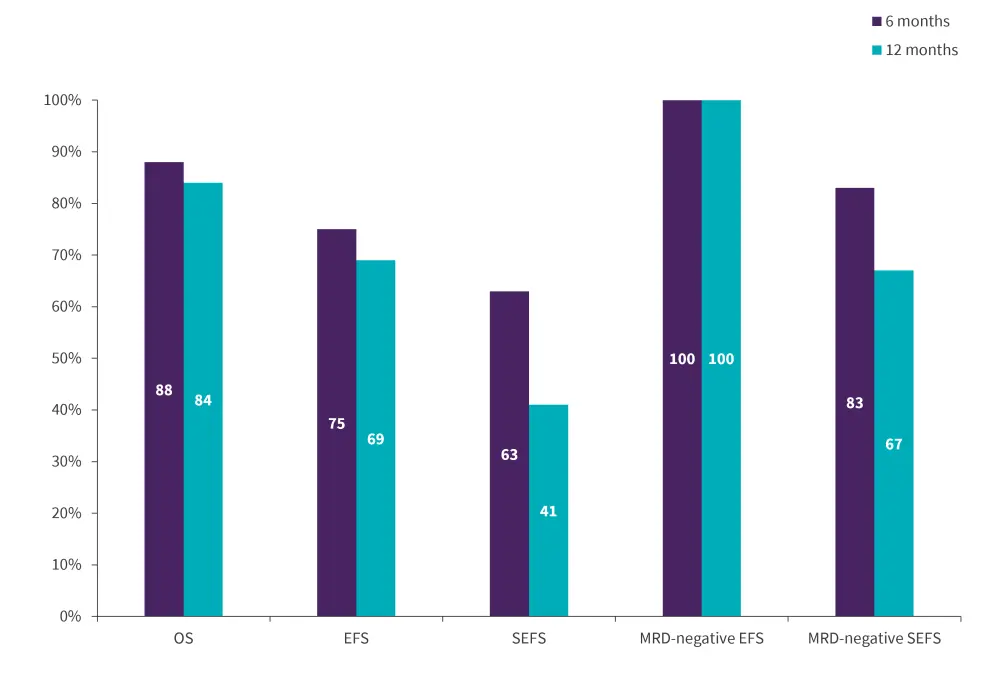
EFS, event-free survival; MRD, measurable residual disease; OS, overall survival; SEFS, stringent EFS.
*Data from Ghorashian, et al.1
Safety
- Cytokine release syndrome (CRS) of any grade occurred in 21 patients who received a tisa-cel infusion, with five patients experiencing ≥Grade 3 CRS
- The median duration of CRS was 1.5 days, in comparison to 8 days in the ELIANA trial
- Neurotoxicity or immune effector cell-associated neurotoxicity syndrome occurred in nine patients; however, no patients experienced severe neurotoxicity
- Prolonged cytopenia occurred in 15 patients, with 10 experiencing ≥Grade 3 cytopenia
- Infection occurred in ten patients, with nine experiencing a ≥Grade 3 infection
- Febrile neutropenia occurred in two patients
- The probability of ongoing B-cell depletion at 6- and 12-months was as 88% and 70%, respectively
- Hypogammaglobulinemia occurred in 87% of patients, all of whom were treated with immunoglobulin replacement therapy in accordance with local protocol
Conclusion
The results of this study provide vital real-world data on the application of tisa-cel therapy in patients aged ≤3 years. The data indicates a safety and efficacy profile largely consistent with that observed in older children or young adults in the ELIANA trial. Responses were durable and sustained in many cases. While the findings are encouraging, long-term follow-up data is required to sufficiently inform treatment protocols in this difficult-to-treat patient population.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


