All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Tisagenlecleucel vs standard of care in children and young adults with R/R ALL
Question 1 / 1
In the study by Stackelberg et al., what was the 2-year adjusted overall survival (OS) rate associated with tisagenlecleucel in the intention to treat analysis (OSITT)?
A
59.49
B
60.38
C
36.16
D
48.21
The efficacy and safety of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (ALL) have been evaluated in multiple studies, including ELIANA ( NCT02435849), ENSIGN (NCT02228096), and a phase IIIb study (NCT03123939). These trials provided encouraging evidence for CD19+ chimeric antigen receptor T-cell therapy in patients with R/R ALL; however, due to the rarity and severity of ALL, and the ethical challenges of conducting a randomized controlled trial, there is currently no comparative data available.
In this article, we summarize the results of a study published in Leukemia by Stackelberg et al.1 comparing the efficacy of tisagenlecleucel vs standard of care (SOC).
Study design1
This was a retrospective cohort study comparing tisagenlecleucel with SOC in pediatric and young adult patients with R/R B-cell ALL. The data from three pivotal studies (tisagenlecleucel group) was compared with three patient registries from Germany/Austria (historical control arm; SOC group). Figure 1 shows a detailed overview of the data sources used for the analysis.
Figure 1. Overview of the studies and registries included in the tisagenlecleucel vs SOC analysis*
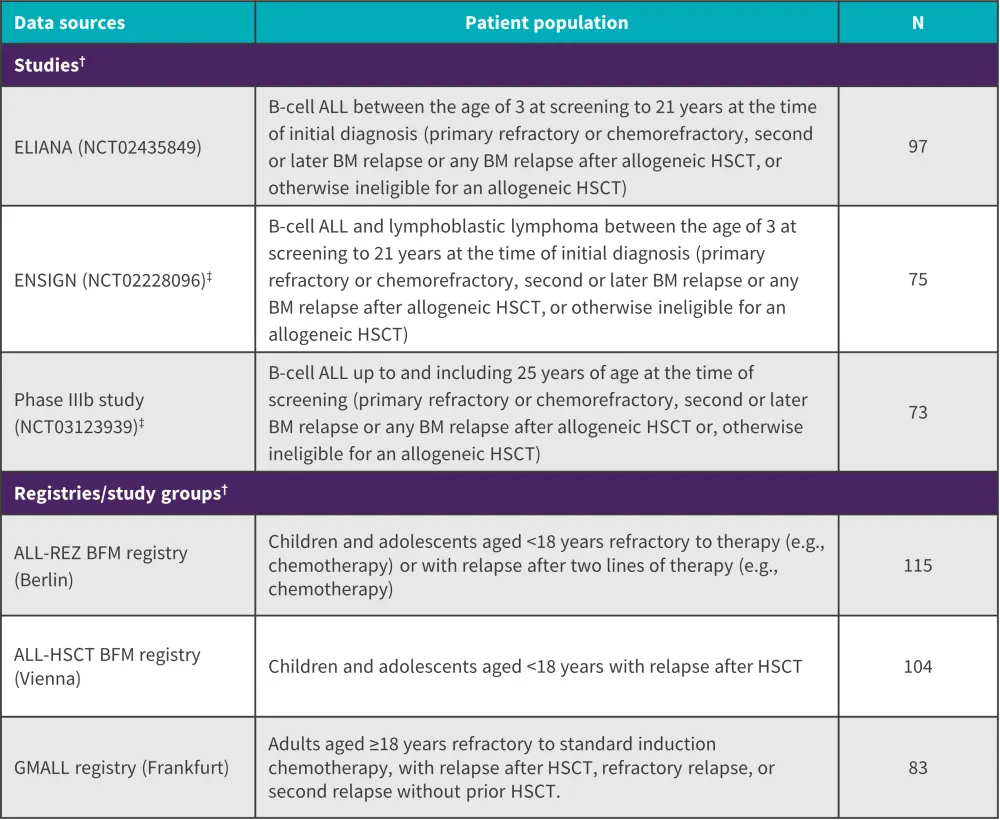
ALL, acute lymphoblastic leukemia; BFM, Berlin-Frankfurt-Münster; BM, bone marrow; EMA, European Medicines Agency; GMALL, German Multicenter Study Group for Adult ALL; HSCT, hematopoietic stem cell transplantation; LTFU, long-term follow up.
*Adapted from Stackelberg, et al.1
†Time periods: ELIANA: data cut from July 1, 2019; ENSIGN-LTFU: final data cut from May 24, 2019, data cut from May 2020 for EMA submission; phase IIIb study-LTFU: data until last patient’s last visit on October 13, 2020, data cut from May 2020 for EMA submission; ALL-REZ BFM: included until September 2017, with the longest possible follow-up period, but at least until the end of 2019; ALL-HSCT BFM: recruitment until 2013 with longest follow-up period, data cut from 2017; GMALL: included until September 2017, with the longest possible follow-up period, but at least until the end of 2019.
‡Including data from LTFU for ENSIGN (n = 31) and the phase IIIb study (n = 42).
Endpoints
Efficacy endpoints included overall survival (OS), event-free survival (EFS), relapse-free survival (RFS), and overall remission rate. The treatment effects were assessed using a:
- full analysis set (FAS), which refers to patients infused with tisagenlecleucel; and
- intention-to-treat (ITT) population, which refers to patients enrolled in tisagenlecleucel studies, including those who subsequently received tisagenlecleucel infusion as well as those who did not.
Results1
A total of 243 ITT patients were included in the naïve setting; 209 patients received a tisagenlecleucel infusion (86% of the FAS) and 302 patients received SOC. A total of 201 patients in the tisagenlecleucel population and 273 patients in the SOC population were included in the FAS. The baseline confounders were well balanced between the two populations after adjustment.
Overall response
When compared with the SOC group, tisagenlecleucel was associated with a significantly higher overall response (OR), as indicated by an adjusted ORITT of 1.99 (95% confidence interval [CI], 1.33–2.97; p < 0.001) and ORFAS of 3.34 (95% Cl, 2.14–5.19; p < 0.001).
Overall survival
A significant OS benefit was seen in the tisagenlecleucel group with an adjusted hazard ratio (HR)ITT of 0.54 (95% Cl, 0.41–0.71; p < 0.001) and HRFAS of 0.47 (95% Cl, 0.35–0.62; p < 0.001) vs SOC at 2 years (Figure 2). For OSITT, the median follow-up was 22.5 months for tisagenlecleucel vs 60.5 months for SOC; the median follow-up for OSFAS was 30.2 months (range, 28.1–34.9 months) vs 60.5 months (range, 48.2–75.3 months) in the tisagenlecleucel and SOC group, respectively.
Figure 2. 2-year OS rates in tisagenlecleucel vs SOC group*
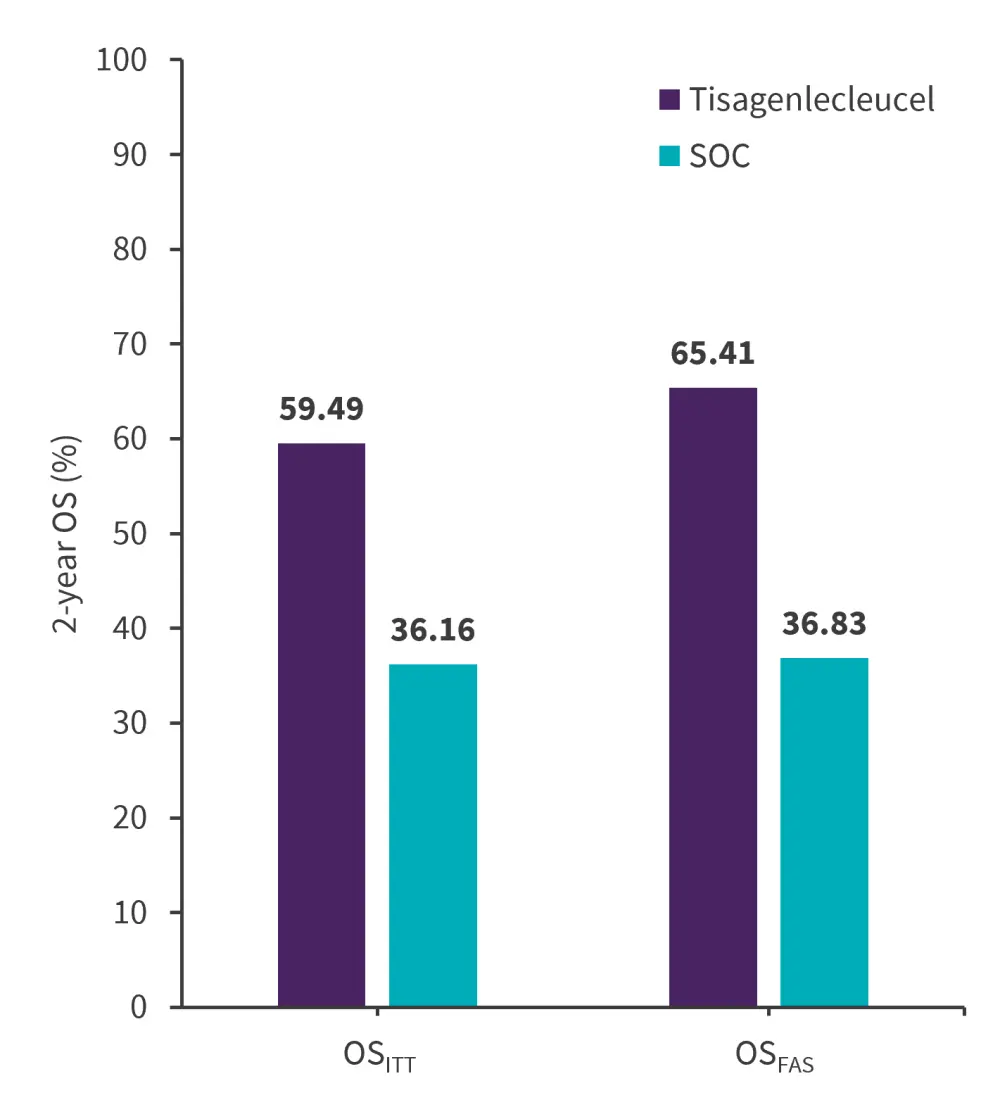
FAS, full analysis set; ITT, intention-to-treat; OS, overall; SOC, standard of care.
*Data from Stackelberg, et al.1
EFS and RFS
Due to limited availability from the registries, confounders could not be adequately balanced for EFS and RFS; therefore, the results of the naïve comparison were presented instead.
For EFSFAS, the naïve median follow-up was 21.2 months for tisagenlecleucel vs 69.9 months for SOC. There was a 33% significantly lower risk of an event in the tisagenlecleucel vs SOC group with an HRFAS of 0.67 (0.52–0.86).
For naïve RFS, the naïve median follow-up was 13.7 (11.3–21.4) months and 73.7 (59.1–102.2) months for tisagenlecleucel vs SOC, respectively, and tisagenlecleucel therapy was non-significantly associated, with a HR FAS of 0.77 (0.51–1.18). The 2-year naïve EFS and RFS rates are reported in Figure 3.
Figure 3. 2-year EFS and RFS rates in tisagenlecleucel vs SOC group*
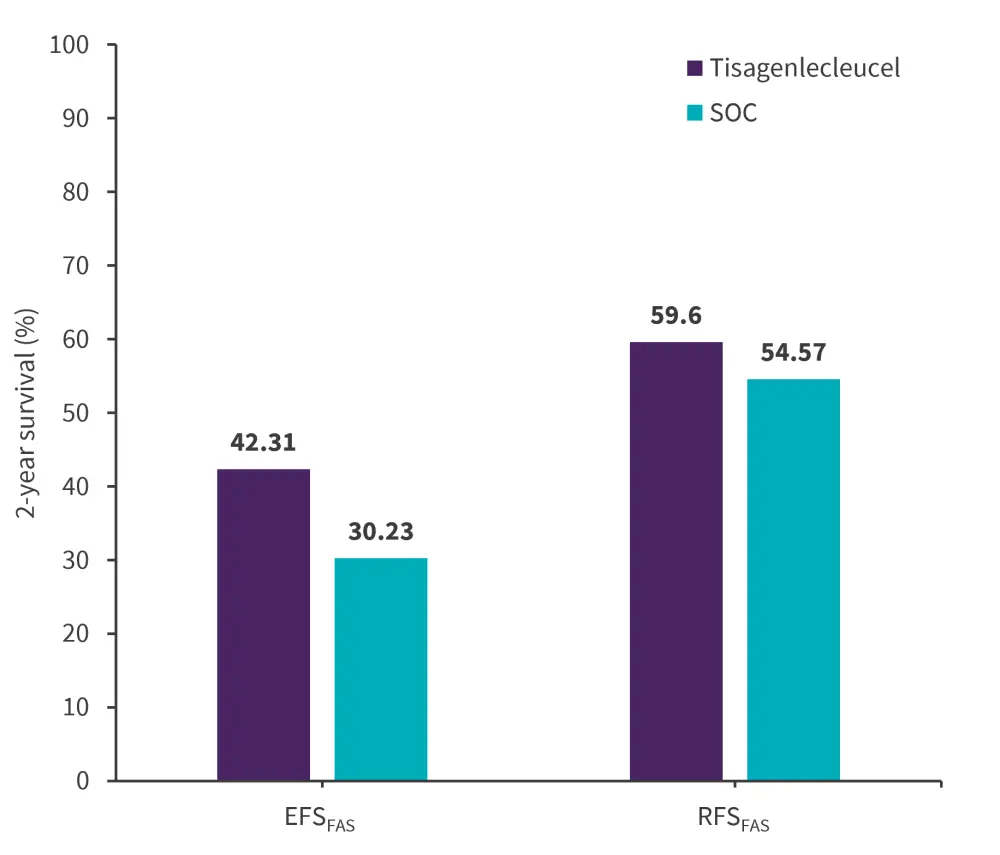
EFS, event-free survival; FAS, full analysis set; RFS, relapse-free survival; SOC, standard of care.
*Data from Stackelberg, et al.1
Conclusion
This retrospective cohort study showed a significant OS benefit with tisagenlecleucel. Overall, the findings support existing evidence that tisagenlecleucel is superior to SOC in pediatric and young adult patients with R/R ALL. However, there were several limitations, such as the limited response and relapse data available for infused patients, lack of EFS data from the German Multicenter Study Group for Adult ALL registries, lack of RFS data from both ALL-hematopoietic stem cell transplantation Berlin-Frankfurt-Münster and adult German Multicenter Study Group for Adult ALL registries, and lack of safety and health-related quality of life outcomes.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


