All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
2023 ELN recommendations Part 2: Clinical management of adult ALL
The European Working Group of the European Leukemia Net (ELN) identified the clinical management of adults with acute lymphoblastic leukemia (ALL) as an unmet need.1
The ALL Hub is pleased to present a summary of the second and final part of the 2023 European LeukemiaNet (ELN) recommendations, published by Gokbuget et al.1 in Blood. Part one was focused on the diagnosis, prognosis, and assessment of adult ALL. This final update is focused on the clinical management of ALL, including new immunotherapies, application of minimal residual disease (MRD), management of specific subgroups, late effects, and supportive care. 1
Application of MRD in treatment decisions1
- Key factors guiding MRD-based treatment decisions include time-point, level, and method of MRD detection and presence of other prognostic factors.
- Earlier time points could help to identify patients for de-escalation therapy
- The current threshold for clinical decisions is a sensitivity level of 0.01% or 10-4
- Quantitative polymerase chain reaction warrants a 4–6 week set-up for appropriate assay, whereas multi-flow cytometry can deliver faster but more uncertain results
- In patients with persistent or recurrent MRD, a change in therapy is recommended.
- Patients with MRD >0.01% after three blocks of standard therapy can be given stem cell transplantation (SCT) or targeted therapies.
Treatment recommendations for specific subgroups1
Adolescent and young adults (15–20 years) and older patients (>55–65 years)
- For adolescents and young adults, pediatric-based protocols are used until ages 55-65 years.
- For older patients, age-adapted protocols can achieve complete remission (CR) rates of 43–90% and overall survival <30–40%; pre-phase therapy is critical for management.
Philadelphia chromosome-negative ALL
- Immunotherapies such as rituximab, blinatumomab, and inotuzumab ozogamicin (InO) have demonstrated promising efficacy and have been incorporated in frontline trials (Figure 1).
- Rituximab for at least eight doses is recommended for the treatment of CD20+ ALL.
- Participation in InO and blinatumomab trials is encouraged for newly diagnosed patients with CD22+ and CD19+ B-ALL, respectively.
- For older patients, SCT is not recommended for MRD-negativity outside of clinical trials.
Philadelphia chromosome-positive ALL
- Tyrosine kinase inhibitors (TKIs) achieve high CRs, low toxicity, and allow a higher proportion of patients to undergo SCT (Figure 1); therefore, it should be given as early as possible.
- Choice of first-line TKI depends on treatment regimen and comorbidities
- A high initial dose of imatinib (800 mg/day for 6–8 weeks) is linked to better outcomes.
- Reducing ponatinib dose (45–30 mg/day) when CR is reached lowers the risk of arterial occlusive events without affecting efficacy.
- Combining TKIs with immunotherapy could further improve clinical efficacy
- SCT remains the standard of care, but its role in the era of third-generation TKIs could be limited
Figure 1. Selected frontline trials in Ph- ALL and Ph+ positive ALL
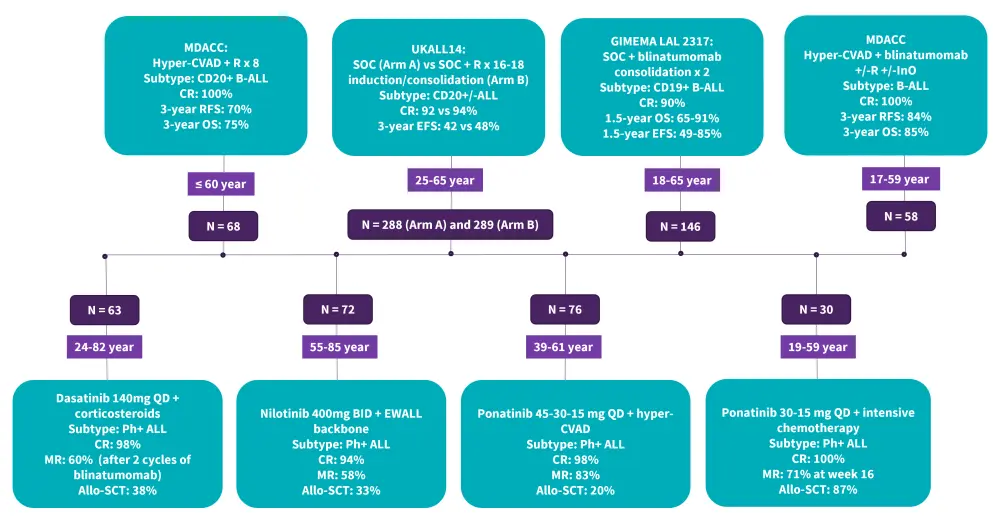
ALL, acute lymphoblastic leukemia; Allo-SCT, allogeneic stem cell transplantation; BID, twice a day; CR, complete remission; EFS, event-free survival; EWALL, European Working Group for Adult Acute Lymphoblastic; GRAALL, Group for Research on Adult Acute Lymphoblastic Leukemia; Hyper-CVAD, hyperfractionated-cyclophosphamide, vincristine, doxorubicin, and dexamethasone; MDACC, MD Anderson Cancer Center; MR, molecular response; OS, overall survival; Ph+, Philadelphia chromosome-positive; Ph- Philadelphia chromosome-negative; QD, once daily; R, rituximab; RFS, relapse-free survival; SOC, standard of care.
*Data from Gokbuget, et al.1
Philadelphia chromosome-like ALL
- Treatment of Philadelphia (Ph)-like ALL remains a challenge, but novel therapies such as blinatumomab and InO have demonstrated encouraging efficacy.
- Due to poor MRD response, most groups rely on an MRD-based approach and will be offered SCT and/or targeted therapy.
T-cell ALL
- Participation in nelarabine trials is recommended until concrete data is available
- ETP ALL has a poor prognosis, while chemotherapy and unfavourable oncogenetics should also lead to consideration of SCT
- Older patients with high-risk T-ALL could benefit from reduced-intensity conditioning if they have a well-matched donor
Relapsed/refractory ALL
- All patients with relapsed/refractory (R/R) ALL should be referred to experienced centers and considered for SCT
- The choice of regimen will depend on several factors, including age, comorbidities, disease characteristics, type of prior therapy, and duration of prior remission
- Patients with extramedullary relapse should be given CNS-directed treatments
- Patients with isolated or combined CNS relapse should be directed to SCT
- Clinically approved immunotherapies such as blinatumomab, InO, and chimeric antigen receptor T-cell have demonstrated efficacy in R/R ALL (Figure 2)
- The indication for chimeric antigen receptor-T in relapse after SCT is generally accepted, even in the setting of MRD relapse
Figure 2. Selected trials in R/R B-ALL*
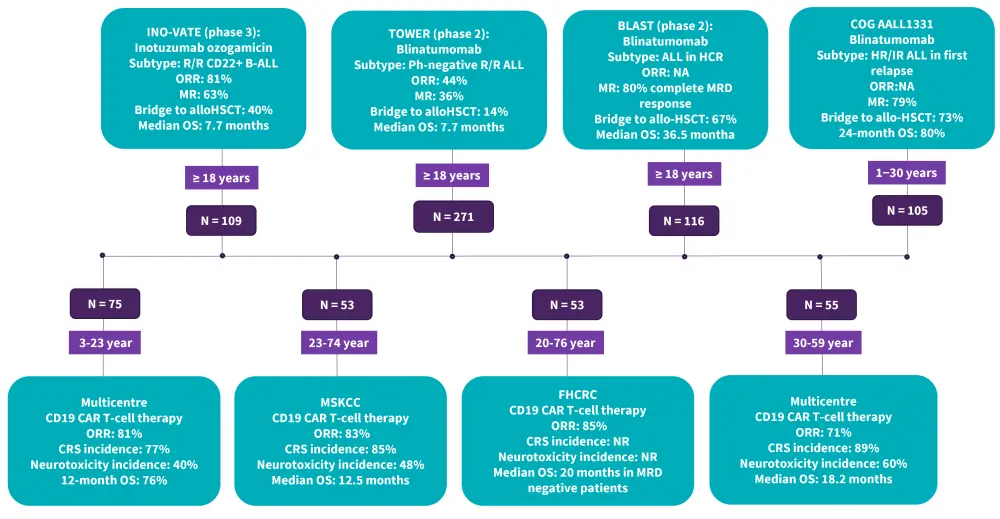
B-ALL, B-cell acute lymphoblastic leukemia; Allo-SCT, allogeneic stem cell transplantation; CRS, cytokine release syndrome; FHCRC: Fred Hutchinson Cancer Research Center; HCR, hematologic complete remission; HR, high risk; IR, intermediate risk; MRD, minimal residual disease; MSKCC: Memorial Sloan Kettering Cancer Center; MR, molecular response; NA, not available; NR, not recorded; ORR, overall response rate; OS, overall survival; Ph, Philadelphia chromosome
*Data from Gokbuget, et al.1
ALL in pregnancy
- ALL in pregnancy should be managed in experienced centers
- Treatment options will be based on gestational age, disease biology, and clinical status
- Termination of pregnancy is strongly considered in the first trimester.
- Systemic antifolates should not be used at any time during pregnancy.
Down syndrome ALL
- Reduction of anthracyclines is recommended for down syndrome ALL
- Prophylactic management of infections is strongly recommended
Late effects1
- Late effects in ALL can include thyroid disorders, infertility, osteonecrosis, cardiotoxicities, neuropsychological disorders, fatigue, and secondary malignancies.
- Avascular osteonecrosis is most commonly observed in adolescents/young adults
- Cardiovascular check-ups should be performed in the aftercare of patients
- Risk factors linked to secondary malignancies include alkylating agents, central nervous irradiation, total body irradiation before SCT, and different gene mutations
Supportive care1
- Organ damage due to very high white blood cell counts can be treated with leukapheresis
- Patients at high risk of developing tumor lysis syndrome should be given hydration
- Antibacterial, antifungal, and antiviral prophylaxis for infection management is essential
- Sperm and ovarian tissue cryopreservation is encouraged in men and women to preserve fertility.
| Key learnings |
|
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

