All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Blinatumomab combined with dasatinib and steroids in older patients with Philadelphia-positive ALL: Results from a phase II study
The adults with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) have shown improved outcomes with tyrosine kinase inhibitor (TKI) based therapy. However, many older patients who are not good candidates for intensive chemotherapy are treated with a combination of TKIs and corticosteroids. Although the remission rate with TKI/steroid-based therapy is high, the short median disease-free survival (DFS) remains a limitation.1
Blinatumomab, an anti-CD19 bispecific T-cell-engaging antibody, has emerged as a novel therapy in the treatment of relapsed/refractory (R/R) B-cell ALL and measurable residual disease (MRD)-positive B-cell ALL, as previously published on the ALL Hub. Its use in combination with TKIs, such as ponatinib, has also been studied in adult patients with newly diagnosed and R/R Ph+ ALL Ph+ ALL, as previously published on the ALL Hub; however, the feasibility of this combination in older patients with Ph+ ALL is not well known.
To address this knowledge gap, Advani et al.1 recently published an article in Blood Advances evaluating the feasibility and outcomes of combining blinatumomab with the TKI dasatinib and prednisone in older patients with newly diagnosed Ph+ and Ph-like ALL. The key findings are summarized below.
Methods1
This is an ongoing open-label phase II trial (NCT02143414) to assess safety and feasibility of dasatinib/prednisone induction followed with blinatumomab/dasatinib in older patients with ALL.
Eligible patients were
- ≥65 years old with Ph+ or Ph-like ALL (with dasatinib-sensitive fusions/mutations);
- newly diagnosed or R/R with no evidence of central nervous system (CNS) disease;
- no active pericardial or pleural effusion;
- no history or presence of clinically relevant CNS pathology;
- cardiac ejection fraction of ≥45%; and
- adequate organ function.
The induction therapy consisted of dasatinib/prednisone followed with post-remission therapy (PRT) with blinatumomab/dasatinib and maintenance with dasatinib/prednisone. The detailed treatment schema is illustrated in Figure 1. The response was assessed at Days 28, 56, and 84; additional time points were dependent on response. MRD was assessed centrally by 8-color flow cytometry at Day 28 and MRD negativity was defined as <0.01%.
Figure 1. Treatment schema*
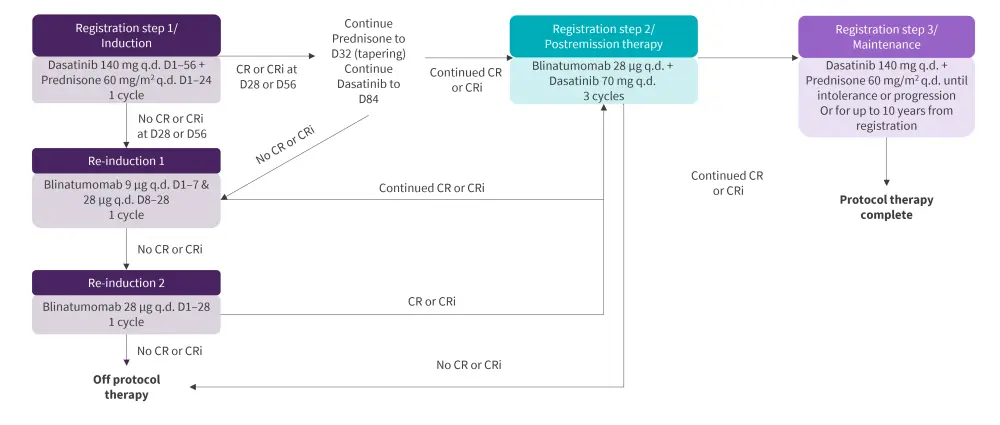
CR, complete remission; CRi, CR with incomplete count recovery; D, day; q.d., once daily.
*Adapted from Advani, et al.1
The primary objective was to assess the feasibility (safety) of dasatinib/blinatumomab combination treatment in older patients with Ph+ ALL. Secondary objectives included estimated overall survival (OS), DFS, and the rate of MRD negativity.
Results1
Of 12 patients receiving PRT and evaluable for dose-limiting toxicities (DLTs), four patients experienced DLTs including Grade 3 hypertension (n = 1), Grade 3 dyspnea and gastrointestinal pain (n = 1), Grade 3 dyspnea (n = 1), and Grade 3 hyperglycemia (n = 1). The DLTs were deemed acceptable following National Cancer Institute (NCI) and the Food and Drug Administration (FDA) review and the study reopened for further accrual.
Overall, 24 patients with newly diagnosed Ph+ ALL and a median age of 73 years (range, 65–87 years) were accrued. The key baseline characteristics are listed in Table 1.
Table 1. Patient baseline characteristics*
|
*Adapted from Advani, et al.1 |
|
|
Characteristic, % (unless otherwise stated) |
N = 24 |
|---|---|
|
Patients with additional cytogenic abnormalities |
79 |
|
Male |
33 |
|
Race |
|
|
Asian |
8 |
|
Black |
4 |
|
White |
75 |
|
Unknown |
13 |
|
Hispanic ethnicity |
|
|
Hispanic |
13 |
|
Not Hispanic |
75 |
|
Unknown |
13 |
|
Median baseline white blood cell count (range), ×1,000 |
7.5 (1.3–123.3) |
|
Median bone marrow blast (range), % |
89 (30–100) |
Toxicities1
During induction, treatment-related nonhematologic Grade 4 toxicities occurred in two patients. During PRT or maintenance, no Grade 4 or higher treatment related nonhematologic toxicities were observed. The hematologic and nonhematologic toxicities during PRT are shown in Figure 2.
Figure 2. PRT A nonhematologic and B hematologic AEs possibly related to treatment*
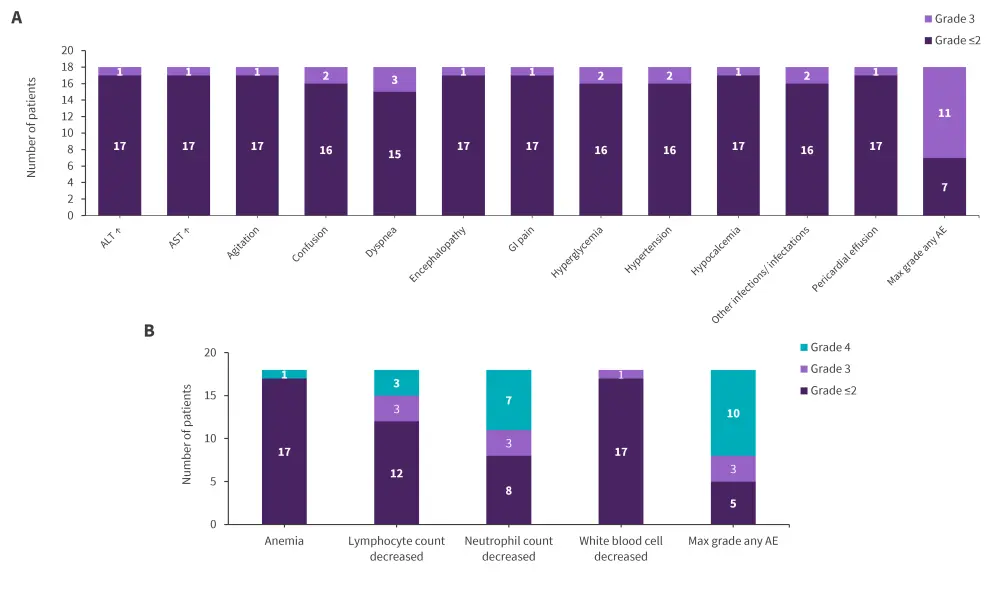
ALT, alanine transaminase; AST, aspartate aminotransferase; AE, adverse event; GI, gastrointestinal; PRT, post-remission therapy.
*Data from Advani, et al.1
Response/Outcomes1
The Consolidated Standards of Reporting Trials diagram of patients is shown in Figure 3. MRD data was available for 16 of 22 patients achieving a complete response CR, of which six patients were MRD negative by flow cytometry at Day 28. Of 19 patients analyzed, 17 were in major molecular or CR at some point after treatment, with at least 12 patients achieving CR. Of seven patients with detectable transcript at the end of induction, five patients achieved CR after blinatumomab-based PRT.
With a median follow-up of 2.7 years, 3-year OS was 87% (95% confidence interval [CI], 64–96) and DFS was 77% (95% CI, 54–90).
Figure 3. Consolidated Standards of Reporting Trials diagram*
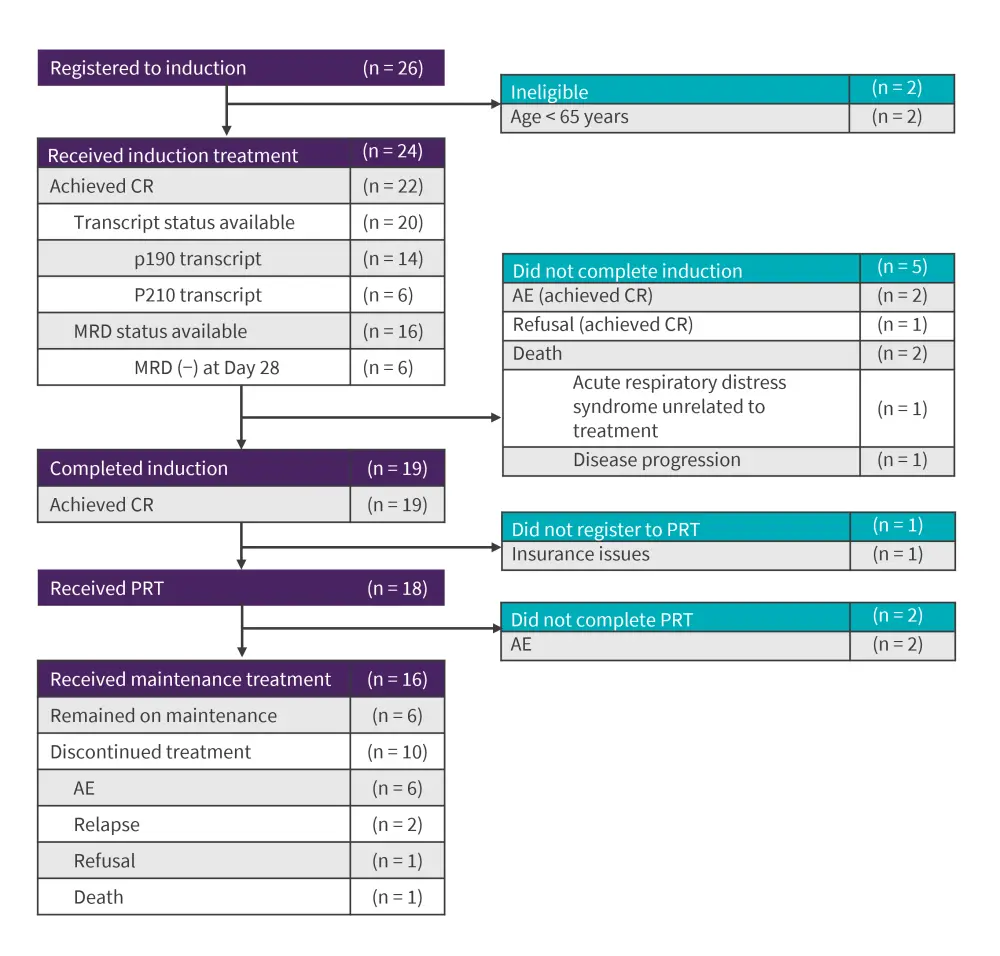
AE, adverse event; CR, complete remission; MRD, measurable residual disease; PRT, post-remission therapy.
*Adapted from Advani et al.1
Conclusion1
The authors concluded that the combination of dasatinib and blinatumomab was well tolerated and had impressive OS and DFS in older patients with Ph+ ALL. However, a longer follow-up is warranted to determine the duration of these responses. In addition, as the current approach did not include transplantation, further studies are needed to assess response in younger patients, who are often offered transplant in first CR.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content




