All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
CNS leukemia in pediatric ALL: mechanisms, diagnostics, and treatment
Central nervous system (CNS) leukemia is clinically characterized by the presence of leukemic cells in the cerebrospinal fluid (CSF).1 Although the introduction of CNS-directed chemotherapy has significantly improved survival rates in childhood acute lymphoblastic leukemia (ALL), CNS involvement accounts for 30–50% of relapses and is associated with immediate, intermediate, and long-term toxicities, thus these are major clinical concerns.2
Given the burden of CNS involvement, there is an increasing need to understand the molecular mechanisms, identify diagnostics and potential biomarkers, and optimize CNS treatment for better disease control and reduced toxicity.2,3,4
The ALL Hub has previously reported on CNS involvement at diagnosis in childhood ALL and the management of CNS disease in adults. Here, we summarize three key presentations on the molecular mechanisms, diagnostic and potential biomarkers of CNS leukemia, and clinical consequences of CNS treatment, presented at the European Hematology Association (EHA) 2023 Congress.
Molecular mechanisms of CNS leukemia2
Izraeli outlined the molecular mechanisms of CNS leukemia, highlighting the types of cells, the routes of entry, two-or one-way traffic, colonization and adaptation of CNS ALL cells, as well as potential novel therapeutics under investigation.
Access to the CNS: Types of cells and routes of entry
Preclinical data have elucidated two possible models for CNS leukemia. The first proposes that every ALL cell can migrate to the CNS, but only cells with activated survival pathways cause CNS leukemia. The second model suggests that only ALL cells with up-regulated homing markers, such as CXCR4 or CCR7, can migrate to the CNS and activate the necessary survival pathways to maintain CNS disease. Data from xenograft models have shown selective localization to the bone marrow (BM) and extramedullary sites; however, another study has demonstrated no evidence of selective trafficking to the CNS.
The main entrance route of CNS blasts into the CNS is through blood vessels which link the bone marrow to the subarachnoid space, mediated by integrin alpha 6 (α6) adhesion molecules. These have been linked to both migration and minimal residual disease (MRD) in ALL. The anti-human α6-blocking antibody P5G10 induced cell death and sensitized primary ALL cells to chemotherapy and tyrosine kinase inhibitors in vivo and in vitro; thus it is being evaluated as a novel therapeutic target in B-cell ALL (B-ALL).
A CNS-specific study by Meyer et al. found that high expression of vascular endothelium growth factor (VEGF) on ALL cells facilitated migration to the CNS. The anti-VEGF antibody bevacizumab significantly reduced CNS leukemia and CNS colonization in vivo, and therefore remains a promising novel CNS strategy. Functional lymphatic vessels were discovered within the dural sinuses, shedding light on the possibility of two-way traffic in CNS involvement.
In the CNS: CNS colonization, and adaptation
A 2007 study by Cario et al. showed that cytokine interleukin (IL)-15 is implicated in the risk of CNS disease, both at diagnosis and relapse. Pediatric mice injected with S49 cells expressing IL-15 resulted in CNS symptoms, confirming the role of IL-15 in CNS lymphoma. It was hypothesized that IL-15 can activate natural killer cells to limit the growth of peripheral leukemia without affecting the CNS, allowing time for CNS infiltration and CNS symptoms; to establish these findings, natural killer depletion enhanced peripheral leukemia in mouse models. Further to this, IL-15 promotes cell growth under low serum conditions, mimicking the lack of nutrient supply in the CSF microenvironment.
CNS blasts upregulate fatty acid synthesis and downregulate fatty acid oxidation for survival in the CSF. One such pathway is overexpressing de-stearoyl-CoA, an enzyme typically involved in unsaturated fatty acid synthesis, to enhance CNS leukemia; inhibition of this molecule prevents CNS colonization. Recent studies have also reported the upregulation of cholesterol and protein synthesis pathways in CNS leukemia. Overall, there are different pathways and molecules involved in CNS infiltration and survival (Figure 1).
Figure 1. Pathways and molecules involved in CNS infiltration and survival*
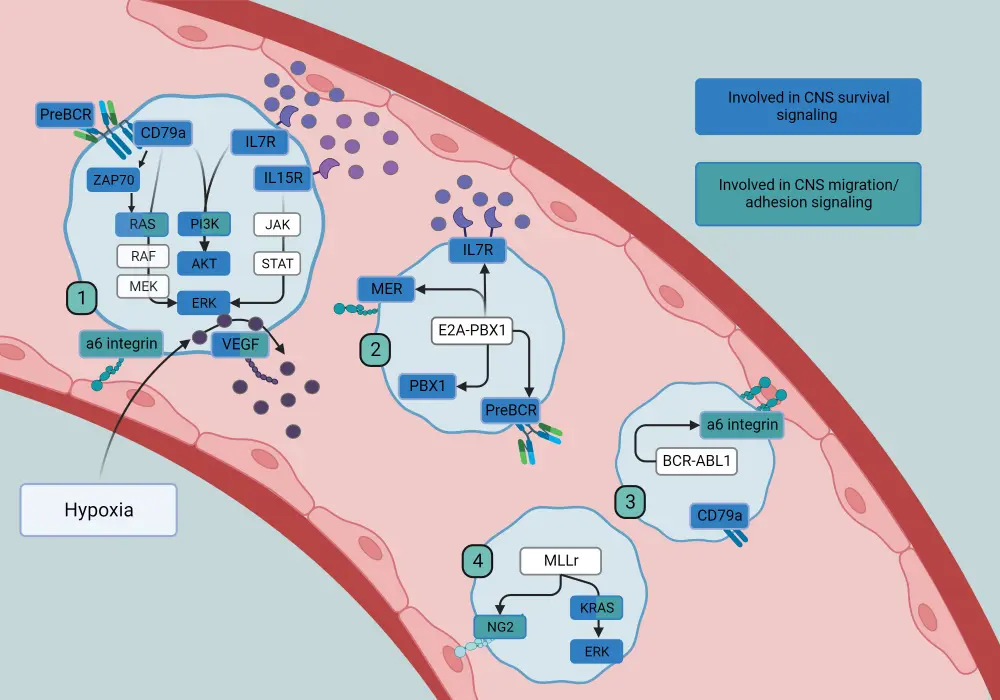
CNS, central nervous system
*Adapted from Lenk.5
Diagnostics and potential biomarkers in CNS leukemia3
Halsey highlighted key diagnostic tools, potential prognostic and response biomarkers in CNS leukemia.
Diagnostics, prognostic, and predictive biomarkers
The diagnosis of CNS leukemia by conventional CSF cell count and cytospin can classify CNS staging (Figure 2); using this method, CNS-3 has been detected in 5% and CNS2/3 in 15–20% of children. Flow cytometry (FC) and polymerase chain reaction are more sensitive methods which can detect CNS blasts in the CSF in up to 35% of children. Clinical evidence reveals that CNS involvement at diagnosis occurs in up to 75%, with preclinical evidence supporting higher rates of CNS disease.
Figure 2. CNS involvement classification at diagnosis*
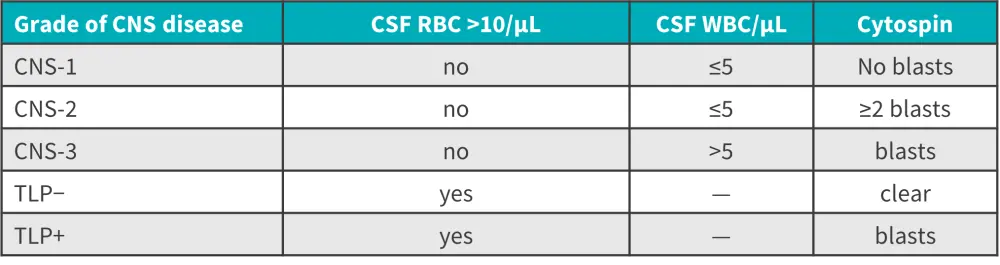
CNS, central nervous system; CSF, cerebrospinal fluid; RBC, red blood cell; TLP, traumatic lumbar puncture; WBC, white blood cell.
*Data from Halsey.3
Although CNS-3 and CNS-1 disease do not have predictive value, they can serve as prognostic biomarkers, with CNS-3 indicating a high risk of CNS relapse and CNS-1 a lower risk. In the NOPHO 2008 study (NCT00819351) including 673 patients with ALL, those with CNS positivity vs negativity by FC had a higher rate of relapse. Patients with a traumatic lumbar puncture (LP) without CNS blasts had the best prognosis, though this was not specific to and did not capture all CNS relapses.
Conversely, little is known about the predictive and prognostic significance of CNS-2, with a wide variation in rates and prognostic significance across different childhood groups. The day of LP, stabilization of CSF samples and transit time to laboratories are key variables affecting the prognostic value of CNS-2; a delay in the LP sample could significantly reduce the number of CNS blasts. Analytical variables which could impact CNS-2 status are automated vs manual cell counts—the former may have less sensitivity, the volume of CSF cytospin, and cytology vs FC.
Other prognostic factors associated with an increased risk of CNS relapse include T-cell leukemia, infants, certain cytogenetic subtypes (BCR:ABL1 Ph+ ALL, t[1:19] [TCF3-PBX1], KMT2A), and a high white blood cell count.3,4 Overall, prognostic biomarkers have limited clinical utility.
Response and toxicity biomarkers
CSF-MRD is an important response biomarker for analyzing the depth of remission during treatment and determining the level of CNS treatment needed. MRD by FC has shown clearance of leukemic blasts from Day 0 to 15 after diagnosis; however, in a study by Schmiegelow et al., little response and much slower clearance of CNS blasts were observed at relapse. To more accurately detect CNS blasts, risk-stratify patients, and adapt treatment accordingly, soluble candidates such as microRNA’s, metabolomic signatures, and cell-free DNA (cfDNA) are currently being investigated. Although difficult to capture, a small study on cfDNA demonstrated its utility in tracking response to therapy.
Clinical consequences of CNS treatment: disease control and toxicity4
Harila gave an overview of the clinical consequences of CNS treatment, including the role of cranial radiation therapy (CRT), intrathecal (IT) chemotherapy, and high-dose (HD) systemic chemotherapy on disease control and toxicity.
Cranial irradiation therapy
Given the significant cognitive impairments, such as IQ decline, and specific neuropsychological deficiencies associated with CRT over time, it has been omitted in first-line treatment. Other complications of CRT include growth hormone deficiencies, prolactin/adrenocorticotropic hormone deficiency, hypothyroidism, risk of metabolic and cardiovascular diseases, and common secondary tumors such as meningiomas.
A meta-analysis including 16,000 patients with ALL in the frontline setting concluded no overall benefit of CRT versus chemotherapy; however, amongst the CNS-3 subgroup, there was a reduced risk of CNS relapse but no difference in event-free survival (EFS) or overall survival (OS) for those receiving CRT. The randomized EORTC-58832 trial reported similar 25-year EFS and OS rates and significantly reduced toxicity in patients treated without versus with CRT; several studies have shown that CRT omission does not compromise survival outcomes. One study showed poor prognosis after CRT among those with early CNS relapse, with another reporting good prognosis in 80% of patients with late isolated CNS relapse after CRT; therefore, CRT is reserved for relapsed disease.
Intrathecal chemotherapy
In the Children's Cancer Group 1952 trial (NCT00002744) comparing IT methotrexate (MTX) with triple IT (ITT) in standard-risk childhood B-ALL, MTX IT led to a better systemic effect, fewer BM relapses, and improved OS (94% vs 90%, respectively), whereas ITT resulted in better CNS effect and fewer CNS relapses. There was no difference in relapses, OS, and toxicities between ITT and IT MTX among children with high-risk B-ALL in the AALL1131 trial (NCT02883049), thus MTX IT remains the standard of care for CNS prophylaxis.
Other investigative options for IT administration include monoclonal antibodies; rituximab has shown promising activity in childhood patients (n = 25) and alemtuzumab has achieved good responses in adult patients with T-cell disease.
HD MTX is a key component of CNS prophylaxis, usually given at an optimum concentration of >1 umol/L in the CSF, with leucovorin rescue to protect healthy cells, and in a 24-hour infusion at 5 g/m2 of at least four infusions. T-cell ALL requires a higher dose, Capizzi uses a MTX dose of 100–300 mg/m2 without leucovorin, followed by asparaginase. In the randomized AALL0232 trial (NCT00075725) including patients with high-risk B-ALL, HD MTX showed a higher 5-year EFS (80% vs 75%), higher 5-year OS (82% vs 75%), and lower CNS and BM relapses. Conversely, Capizzi resulted in a higher 5-year EFS (91 vs 85%), 5-year OS (93 vs 89%), and fewer BM and CNS relapses in the randomized AALL0434 trial (NCT00408005) including patients with T-ALL.
Systemic chemotherapy
Generally, dexamethasone is a more effective glucocorticoid than prednisolone in CNS treatment, demonstrating better CNS penetration and a longer half-life in the CSF, though it is associated with more behavioral problems, infections, and osteonecrosis. Although pegylated-asparaginase is not a CNS penetrative agent, it is an effective CNS treatment for asparagine depletion compared with erwinase.
Nelarabine has demonstrated promising activity in preventing CNS leukemia in T-ALL and dasatinib serves as a better CNS penetrative agent compared with imatinib; however given the controversial results in preventing CNS relapse, its role in this setting remains uncertain.
Chimeric antigen receptor (CAR) T-cell therapies have yielded promising results in CNS leukemia, with several case reports demonstrating effective clearing of cells from the CNS. Across five clinical trials, they have demonstrated comparable outcomes irrespective of CNS relapse status, high survival rates in children with isolated CNS relapse, and no differences in neurotoxicities depending on CNS status. Real-life data report frequent relapses in up to 40% of patients, poor long-term survival outcomes, including those with isolated CNS relapse, and common reports of neurotoxicities.
Clinical toxicities associated with CNS chemotherapy
There are several acute and chronic neurotoxicities, as well as long-term cognitive effects associated with CNS chemotherapy. Acute neurotoxicities include seizures, MTX stroke-like syndrome, posterior reversible encephalopathy syndrome, and sinuous venous thrombosis. Chronic neurotoxicities include behavioral and learning difficulties, and psychological problems (Figure 3).
Figure 3. Incidence and evidence of toxicities related to CNS chemotherapy*
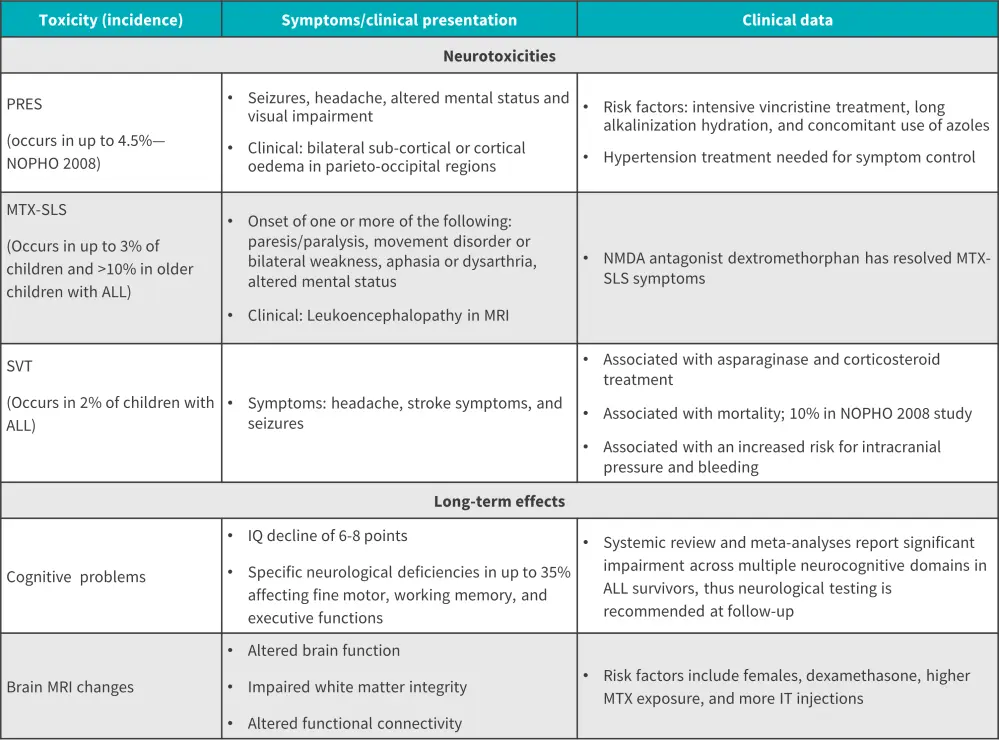
ALL, acute lymphoblastic leukemia; IT, intrathecal; IQ, intelligence quotient; MTX, methotrexate; MRI, magnetic resonance imaging; NMDA, N-methyl-D-aspartate; PRES, posterior reversible encephalopathy syndrome; SLS, stroke like syndrome; SVT, sinus venous thrombosis.
*Data from Harila.4
Future directions and conclusion
Izraeli highlights key mechanisms and components of leukemic blasts, such as integrin α6 adhesion and VEGF molecules involved in migration and adhesion, and IL-15, which aids in the survival of leukemic blasts in the CNS, though more research in this area needed. CNS-1 and CNS-3 are current prognostic and diagnostic biomarkers and CSF-MRD can serve as a response biomarker CNS leukemia; however, Halsey highlights the lack of sensitive biomarkers to accurately measure CNS involvement, risk stratify patients, monitor response to treatment, and direct precision therapy. Investigative targets such as microRNA’s, secreted metabolomic signatures, and cfDNA could accurately define CNS blasts and allow for more accurate risk-stratification. Given the need for consensus and reporting guidelines, an international survey project conducted by Almasi et al. across 30 countries will investigate pre-analytical and analytical variables to standardize CNS diagnostics including CNS-2. Also, the CSF-FLOW study aims to validate biomarkers for CNS leukemia for more accurate relapse-risk prediction and risk-adapted CNS-directed therapy.
Harila described the clinical effectiveness of current systemic chemotherapy, IT chemotherapy, and CRT for CNS disease control and their associated toxicities; Harila and Izraeli both highlighted potential novel therapeutics being investigated, including CAR T-cell therapies, integrin antibodies, P13K inhibitor idelalisib, dasatinib, and fatty acid/cholesterol synthesis inhibitors. Future trials should involve international collaboration with large, shared research data and biobanks, systematic neuropsychological testing, and studies on survivors with late complications.
Ultimately, a better understanding of molecular mechanisms and identification of robust diagnostic, prognostic and response biomarkers will aid in the development of novel directed therapeutic strategies for CNS leukemia that balance disease control and toxicity.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



