All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Comparison of outcomes in adolescent and young adult patients versus younger patients with high-risk B-ALL
Pediatric patients with B-cell acute lymphoblastic leukemia (B-ALL) currently have an overall survival (OS) rate of >90%; however, OS and event-free survival (EFS) rates in adolescent and young adult (AYA) patients remain inferior and variable, attributed in part to the underlying leukemia biology. Historically many AYA patients with B-ALL have been treated using protocols for adult patients which are less intensive than those for pediatric patients. However, AYA survival rates on pediatric-inspired regimens have been higher than for adult protocols and the CALGB 1043 trial1 has reported improved outcomes for AYA patients treated with the prednisone Capizzi (PC) pediatric regimen of the Children’s Oncology Group (COG) AALL0232 trial.
The ALL Hub has previously reported a comparison of toxicities from CALGB 104031 and COG AALL0232 trials2 and more recently an educational theme article outlining the gene expression profiles in AYA and adult patients. Here we present the key findings by Burke, et al.3 published in Leukemia, comparing the outcomes and treatment-related toxicities between AYA and younger patients with high-risk B-ALL treated on the COG AALL0232 trial.
Study design
This was a retrospective cohort study comprising of patients enrolled in the COG AALL0232 trial between 2004 and 2011, with newly diagnosed high risk B-ALL patients, aged 1–9 years with an initial white blood cell (WBC) count ≥50,000/microliter or 10–30 years old with any WBC count. Patients with Down’s Syndrome (DS) were excluded due to increased toxicity. For the purposes of this study, AYA cohort included patients aged 16–30 years.
Patients enrolled in AALL0232 were randomized to receive dexamethasone or prednisone during induction and high dose MTX with leucovorin rescue (HD-MTX) or Capizzi escalating dose MTX (without leucovorin rescue) plus pegaspargase (C-MTX) during interim maintenance (IM) (Figure 1). Early responses were used to stratify patients and included:
- Rapid early responders if they had a morphologic remission marrow (<5% blasts) by induction Day 15 and <0.1% minimal residual disease (MRD) in the bone marrow (BM) at Day 29.
- Slow early responders if they had morphologic remission by Day 29 but had either an M2 (5–25% blasts) or M3 (>25% blasts) BM on induction Day 15 or Day 29 BM MRD ≥0.1%.
Figure 1. Treatment schedule in AALL0232*
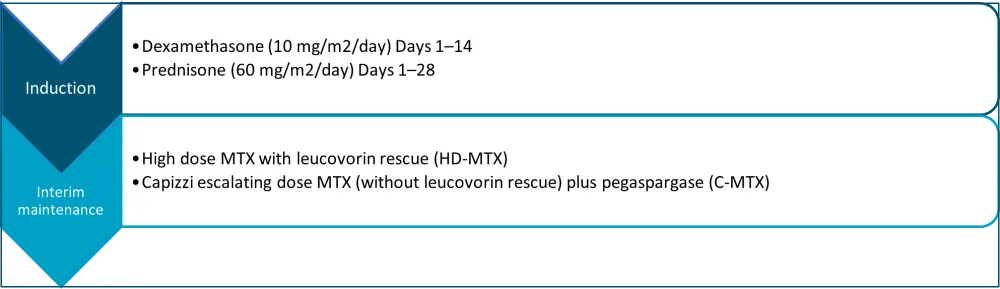
C-MTX, Capizzi escalating dose MTX; MTX, methotrexate.
*Adapted from Burke, et al.4
At post-induction eligible and evaluable patients were grouped into four treatment regimens (Figure 2):
- Prednisone/C-MTX (PC)
- Prednisone/HD-MTX (PH)
- Dexamethasone/C-MTX (DC)
- Dexamethasone/HD-MTX (DH)
Figure 2. Consort diagram*
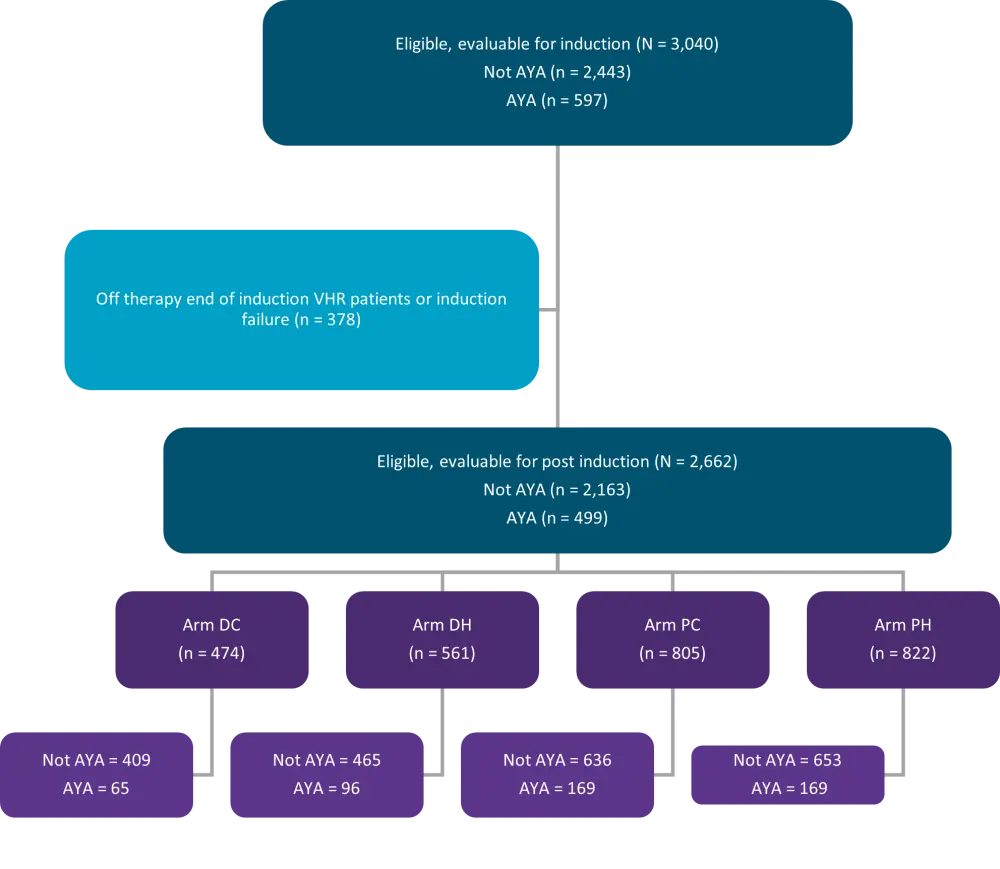
AYA, adolescent and young adults; DC, dexamethasone/C-MTX; DH, dexamethasone/high dose methotrexate with leucovorin rescue; PC, Prednisone/ Capizzi escalating dose MTX (without leucovorin rescue) plus pegaspargase; PH, Prednisone/ high dose methotrexate with leucovorin rescue; VHR, very high risk.
*Adapted from Burke, et al.4
Results
Baseline characteristics
A total of 2,443 patients aged <16 years and 597 aged 16–30 years, were eligible and evaluable for induction therapy. The median age for AYA patients was 17 years and they were more likely to have Philadelphia-like ALL gene expression profile compared to younger patients with National Cancer Institute (NCI) high-risk B-ALL (p = 0.015) and less likely to have ETV6-RUNX1 fusion (p < 0.001) (Table 1).
Table 1. Baseline characteristics*
|
AYA, adolescent and younger adults; BMI, body mass index; MRD, minimal residual disease; Ph, Philadelphia; RER, rapid early responders; SER, slow early responders; WBC, white blood cell. |
|||
|
Characteristics, % (unless otherwise stated) |
Younger (<16 years) n = 2443 |
AYA (≥16 years) n = 597 |
p value† |
|---|---|---|---|
|
Sex, Male |
53.3 |
64.5 |
<0.0001 |
|
Race |
|||
|
White |
84.1 |
89.3 |
|
|
Black or African American |
8.4 |
5.6 |
0.010 |
|
WBC count, ≥50 |
49.5 |
19.6 |
<0.0001 |
|
RER |
80.2 |
66.5 |
|
|
SER |
19.8 |
33.5 |
<0.0001 |
|
MRD induction Day 29 |
|||
|
<0.01 |
73.9 |
55.9 |
|
|
0.01% ≤ MRD<0.1% |
10.0 |
14.7 |
|
|
0.1% ≤ MRD<1.0% |
8.7 |
14.5 |
<0.0001 |
|
1.0% ≤ MRD<10.0% |
5.1 |
10.3 |
|
|
MRD ≥10% |
2.3 |
4.6 |
|
|
BMI ≥30 |
4.8 |
19.5 |
<0.0001 |
|
Ph-like status |
|||
|
Yes |
11.5 |
17.7 |
|
|
No |
88.5 |
82.3 |
0.015 |
|
ETV6-RUNX1 |
|||
|
Yes |
16.4 |
3.8 |
|
|
No |
83.6 |
96.2 |
<0.0001 |
|
Triple trisomy |
|||
|
Yes |
12.4 |
12.0 |
|
|
No |
87.6 |
88.0 |
0.828 |
|
Double trisomy |
|||
|
Yes |
16.1 |
16.2 |
|
|
No |
83.9 |
83.8 |
0.960 |
|
KM2TA (MLL-R) rearrangement |
|||
|
Yes |
3.9 |
4.0 |
|
|
No |
96.1 |
96.0 |
0.899 |
|
Hypodiploidy |
|||
|
Yes |
2.7 |
3.0 |
|
|
No |
97.3 |
97.0 |
0.711 |
|
BCR-ABL1 positive |
|||
|
Yes |
4.9 |
6.3 |
|
|
No |
95.1 |
93.7 |
0.189 |
|
Number of patients completing protocol therapy |
65.8 |
50.3 |
<0.0001 |
Toxicity of therapy
Induction and post-induction toxicities are summarized in Table 2. Rates of Grade ≥3 hyperglycemia (p < 0.0001) and hyperbilirubinemia (p = 0.0007) were higher while those of Grade ≥3 febrile neutropenia (p < 0.0001) were lower during induction in AYA patients compared to the younger patients. Grade ≥3 febrile neutropenia remained lower (p < 0.0001) in AYA patients during the post-induction period, but there were significantly more deaths in AYA patients in remission compared to younger patients (5.4% vs 2.4%, respectively; p < 0.0001).
Table 2. Induction and post-induction toxicities*
|
AYA, adolescent and young adults; IM, interim maintenance. *Adapted from Burke, et al.4 |
|||
|
Toxicities, % (unless otherwise stated) |
Younger |
AYA |
p value† |
|---|---|---|---|
|
Induction |
|||
|
Hyperglycemia |
15.4 |
23.6 |
<0.0001 |
|
Hyperbilirubinemia |
3.7 |
6.9 |
0.0007 |
|
Thrombosis |
1.2 |
1.5 |
0.470 |
|
Pancreatitis |
0.5 |
0.5 |
0.972 |
|
Febrile neutropenia |
13.8 |
7.4 |
<0.0001 |
|
Post-induction |
|||
|
Mucositis |
11.7 |
18.2 |
0.0002 |
|
Peripheral motor |
7.8 |
12.1 |
0.001 |
|
Febrile neutropenia |
56.8 |
45.2 |
<0.0001 |
|
Hyperbilirubinemia |
9.5 |
17.3 |
<0.0001 |
|
Hepatic failure |
0.3 |
1.3 |
0.009 |
Treatment response and outcome
- AYA patients had a significantly higher rate of induction failure compared to younger patients (p < 0.001), including when more a stringent definition of M3 induction failure was applied (p = 0.038) (Table 3).
- The 5-year EFS and OS rates were inferior for AYA patients (65.4% and 77.4%) compared to younger patients (78.1% and 87.3%; p < 0.0001) and were similar when patients with VHR cytogenic features were excluded.
- Cumulative incidence rate of relapse was higher in AYA patients compared to younger patients (p = 0.0006), mainly due to higher cumulative incidence rates of BM relapse ± extramedullary disease (p < 0.0001) (Table 3).
- Obesity was a significant risk factor for inferior EFS irrespective of age; however, obese AYA patients showed significantly lower EFS compared to younger patients (p = 0.006) (Table 3).
- AYA patients showed a significantly higher cumulative incidence of remission deaths compared to younger patients (p < 0.0001) (Table 3).
Table 3. Induction events and cumulative incidence rates by event type*
|
AYA, adolescent and young adults; CNS, central nervous system; EMD, extramedullary disease; SMN, secondary malignant neoplasm. *Adapted from Burke, et al.4 |
|||
|
Events |
Younger (<16 years) |
AYA (≥16 years) |
p value† |
|---|---|---|---|
|
Induction death |
1.6 |
2.2 |
0.366 |
|
Induction failure (per |
3.5 |
7.2 |
<0.001 |
|
Induction death or |
5.2 |
9.4 |
<0.001 |
|
M3 induction failure†† |
1.0 |
2.0 |
0.038 |
|
|
5-year rate ± |
5-year rate ± |
|
|
Relapse |
13.5 ± 0.7 |
18.5 ± 1.7 |
0.0006 |
|
Marrow ± EMD |
9.1 ± 0.6 |
14.0 ± 1.5 |
<0.0001 |
|
Isolated CNS relapse |
3.6 ± 0.4 |
3.9 ± 0.8 |
0.830 |
|
Relapse, other |
0.9 ± 0.2 |
0.6 ± 0.4 |
0.567 |
|
SMN |
0.8 ± 0.2 |
1.0 ± 0.4 |
0.818 |
|
Remission death |
2.4 ± 0.3 |
5.7 ± 1.0 |
<0.0001 |
Univariate and multivariable analyses of risk factors
Significant risk factors for inferior EFS identified in the univariate analysis included age, race, WBC count, end of induction MRD, cytogenetic features, and BMI. These factors retained their significance in the multivariate analysis (Table 4).
Table 4. Multivariate analyses for EFS*
|
BMI, body mass index; CI, confidence interval; EOI, end of induction; HR, hazard ratio; MRD, minimal residual disease; WBC, white blood cell. |
||
|
Parameter |
HR (95% CI) |
p value |
|---|---|---|
|
Age (<16 vs ≥16 years) |
0.77 (0.63–0.96) |
0.018 |
|
Age as continuous variable |
1.04 (1.02–1.06) |
<0.0001 |
|
Race (Black vs White) |
1.46 (1.09–1.95) |
0.011 |
|
WBC count (<50k vs ≥50k) |
0.72 (0.60–0.86) |
0.0003 |
|
EOI MRD (<0.01% vs ≥0.01%) |
0.28 (0.24–0.34) |
<0.0001 |
|
Cytogenetics |
||
|
Neutral vs favorable |
2.39 (1.82–3.15) |
<0.0001 |
|
BMI |
||
|
30–40 vs <30 |
1.67 (1.24–2.26) |
0.0009 |
|
>40 vs <30 |
1.78 (1.01–3.14) |
0.046 |
Conclusion
This retrospective cohort study from COG AALL0232 demonstrated that AYA patients had significantly inferior EFS and OS rates, as well as having higher rates of treatment related toxicity when compared with younger patients. The findings were consistent with studies reported >15 years ago and although treatment intensification strategies have improved outcomes in younger patients, these have not translated into improved outcomes for AYA patients. Further trials with novel approaches to improve outcomes and reduce toxicity in AYA patients are therefore warranted.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

