All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
GRAALL-2005 study: Impact of CNS involvement on outcomes in adult patients with Philadelphia chromosome-negative ALL
A minority (4–11%) of adult patients with acute lymphoblastic leukemia (ALL) have central nervous system (CNS) involvement at the time of diagnosis.1 CNS involvement has been associated with lower overall survival (OS) rates in the MRC UKALL XII/ECOG E2993 trial,2 and involvement of additional extramedullary sites, T-cell ALL, and a higher white blood cell (WBC) count at diagnosis are more common in this subset of patients.1 Since the introduction of pediatric-inspired regimens, the outlook for adult patients with Philadelphia chromosome-negative ALL has significantly improved; however, the effects of initial CNS involvement had not previously been reexamined.1 The ALL Hub previously reported on CNS involvement at diagnosis and its association with CNS toxicities in childhood ALL.
Here, we summarize an article recently published in Haematologica by Orvain et al.1, which investigated the impact of CNS involvement in adult patients with Philadelphia chromosome-negative ALL in the GRAALL-2005 study.
Study design and methods
Patients included in the analysis were:
- aged 18–59 years;
- diagnosed with Philadelphia chromosome-negative ALL between 2006 and 2014;
- treated according to the GRAALL-2005 pediatric-inspired protocol; and
- had diagnostic data on CNS involvement by cerebrospinal fluid.
CNS involvement was categorized as:
- CNS-2: <5 WBC/μl and a positive conventional cytospin; or
- CNS-3: >5 WBC/μl and a positive conventional cytospin and/or clinical signs.
Measurable residual disease (MRD) was monitored by standardized quantitative real-time polymerase chain reaction with a sensitivity of ≥10−4, which was performed after the first induction course.
The aim of the study was to evaluate the impact of CNS involvement on survival outcomes.
Results
Patient characteristics
- Among the 784 adult patients included, 55 had initial CNS involvement, with 47 classified as CNS-3 and 7 as CNS-2 (Figure 1).
- CNS-positive (CNS+) patients were more likely to have T-ALL, leukocytes ≥30 G/l, and higher hemoglobin levels.
- Among patients with clinical symptoms, trigeminal anesthesia was the most prominent clinical characteristic, which was reported in 41%.
- CNS-negative (CNS−) and CNS+ patients showed similar rates of first CR, induction death, and achievement of MRD negativity after induction (Ig/TCR <10−4). Further baseline characteristics are summarized in Table 1.
Figure 1. Flowchart of patients with CNS involvement at diagnosis*
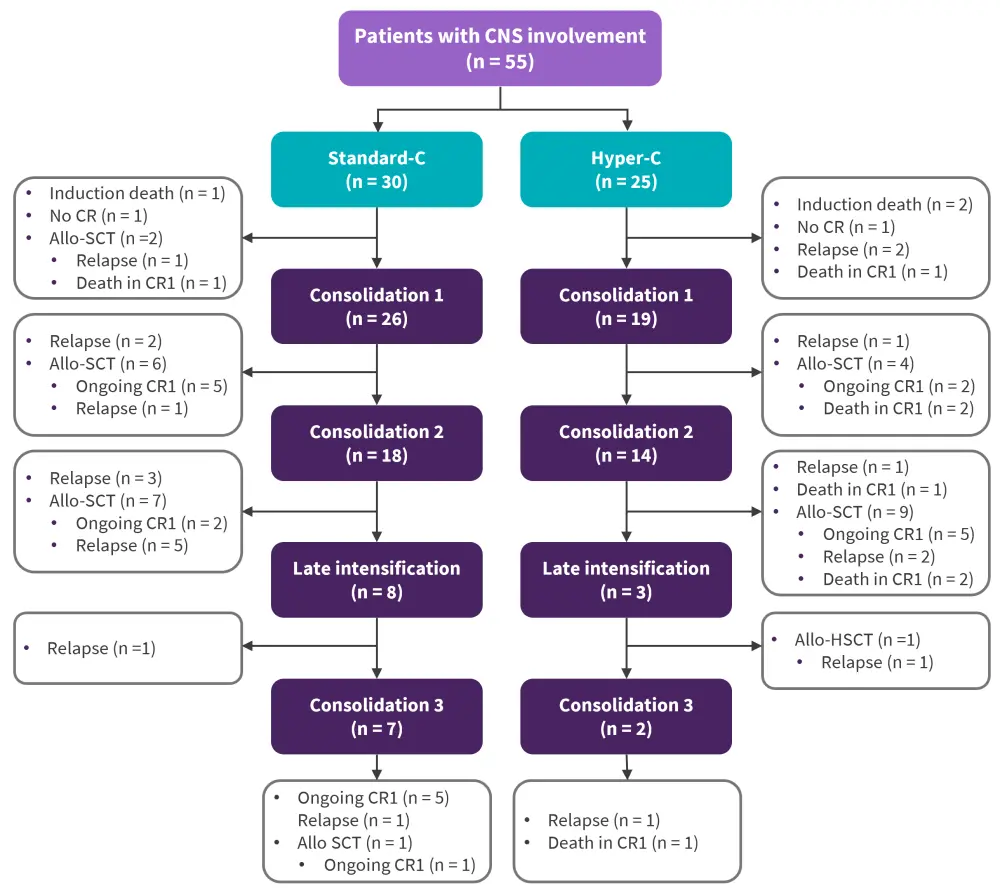
allo-SCT, allogeneic stem cell transplantation; CNS, central nervous system; CR, complete remission.
*Adapted from Orvain, et al.1
Table 1. Characteristics of patients with or without CNS involvement*
|
allo-SCT, allogeneic stem cell transplantation; BM, bone marrow; BMI, body mass index; CNS, central nervous system; CR, complete remission; MRD1, measurable residual disease after induction; PB, peripheral blood; WBC, white blood cells. |
||||
|
Characteristic, % (unless otherwise stated) |
All |
CNS-negative |
CNS-positive |
p value |
|---|---|---|---|---|
|
Median age, years |
36 |
37 |
30 |
0.15 |
|
Female |
40 |
40 |
33 |
0.27 |
|
Median BMI, kg/m2 |
23.6 |
23.7 |
23.5 |
0.75 |
|
Phenotype |
|
|
|
0.004 |
|
B-cell |
67 |
68 |
49 |
|
|
T-cell |
33 |
32 |
51 |
|
|
Median WBC count at diagnosis, G/L |
12 |
11 |
23 |
0.15 |
|
Median hemoglobin level at diagnosis, g/dL |
10.2 |
10.1 |
11.1 |
0.02 |
|
Median platelet count at diagnosis, G/L |
72 |
72 |
78 |
0.16 |
|
Poor early PB blast clearance |
24 |
24 |
27 |
0.50 |
|
Poor early BM blast clearance |
39 |
39 |
38 |
0.58 |
|
CR |
92 |
92 |
91 |
0.79 |
|
Induction death |
6 |
6 |
6 |
0.99 |
|
MRD1 negativity |
16 |
16 |
13 |
0.26 |
|
Allo-SCT in first CR |
35 |
34 |
55 |
0.002 |
Association between CNS involvement and patient outcomes
- Median OS was shorter in CNS+ patients compared with CNS− patients (1.9 years vs not reached; p < 0.001).
- CNS involvement at diagnosis was not associated with a statistically different cumulative incidence of relapse (p = 0.11), but it was associated with significantly higher non-relapse mortality (NRM; p = 0.01). Causes of NRM were similar between CNS+ and CNS− patients, and included infections, transplant-related, thrombosis, bleeding, second cancer, and others.
- The rate of 3-year relapse was higher in CNS+ patients compared with CNS− patients (34% vs 26%, respectively).
- Following censoring patients at allogeneic stem cell transplantation (allo-SCT), the cumulative incidence of relapse was significantly higher in CNS+ patients (p = 0.01), whereas NRM was not different between the two groups (p = 0.4)
Factors associated with survival
As shown in Table 2, univariable Cox regression analysis revealed a positive association between lower OS and age (p < 0.001), body mass index (p < 0.001), leukocytes ≥30 G/L (p = 0.1), CNS involvement (p = 0.001), and poor early BM blast clearance (p = 0.02).
In addition, the multivariable Cox regression model showed a significant association between CNS involvement at diagnosis and lower OS (p <0.001). The only other key variable factors associated with lower OS were age (p < 0.001) and leukocytes ≥30 G/L (p = 0.03; Table 3).
Table 2. Univariable analysis of factors associated with NRM, relapse, and overall survival by Cox regression*
|
BM, bone marrow; BMI, body mass index; CNS, central nervous system; HR, hazard ratio; NRM, non-relapse mortality; PB, peripheral blood; WBC, white blood cells. |
||||||
|
Variable |
NRM |
Relapse |
Overall survival |
|||
|---|---|---|---|---|---|---|
|
HR |
p value |
HR |
p value |
HR |
p value |
|
|
Age/10 |
1.6 |
<0.001 |
1.1 |
0.1 |
1.3 |
<0.001 |
|
Female |
1.4 |
0.06 |
1 |
0.7 |
1.1 |
0.4 |
|
BMI/10 |
1.8 |
<0.001 |
1.1 |
0.4 |
1.4 |
<0.001 |
|
T-cell phenotype |
0.7 |
0.04 |
1 |
0.8 |
0.8 |
0.2 |
|
WBC count at diagnosis >30 G/L |
0.9 |
0.7 |
1.7 |
<0.001 |
1.4 |
0.01 |
|
CNS involvement |
2.1 |
0.01 |
1.5 |
0.1 |
1.8 |
<0.001 |
|
Poor early PB blast clearance |
1.1 |
0.7 |
1.4 |
0.02 |
1.3 |
0.05 |
|
Poor early BM blast clearance |
1.2 |
0.3 |
1.5 |
0.01 |
1.3 |
0.02 |
Table 3. Multivariable analysis of factors associated with NRM, relapse, and overall survival by Cox regression*
|
BM, bone marrow; BMI, body mass index; CNS, central nervous system; HR, hazard ratio; NRM, non-relapse mortality; PB, peripheral blood; WBC, white blood cells. |
||||||
|
Variable |
NRM |
Relapse |
Overall survival |
|||
|---|---|---|---|---|---|---|
|
HR |
p value |
HR |
p value |
HR |
p value |
|
|
Age/10 |
1.5 |
<0.001 |
1.1 |
0.1 |
1.3 |
<0.001 |
|
Female |
1.3 |
0.2 |
0.9 |
0.6 |
|
|
|
BMI/10 |
1.4 |
0.1 |
1.1 |
0.4 |
1.2 |
0.1 |
|
T-cell phenotype |
0.9 |
0.6 |
0.8 |
0.2 |
|
|
|
WBC count at diagnosis >30 G/L |
0.9 |
0.6 |
1.7 |
<0.001 |
1.3 |
0.07 |
|
CNS involvement |
2.8 |
<0.001 |
1.6 |
0.08 |
2.1 |
<0.001 |
|
Poor early PB blast clearance |
1.2 |
0.5 |
1.1 |
0.6 |
1.1 |
0.5 |
|
Poor early BM blast clearance |
1.2 |
0.3 |
1.4 |
0.03 |
1.3 |
0.04 |
Post-remission treatment for patients with CNS involvement
- CNS+ patients were more likely to receive allo-SCT compared with CNS− patients (55% vs 34%; p = 0.002), with a median interval before proceeding to allo-SCT of 170 days vs 142 days, respectively (p = 0.003). A total of 25 CNS+ patients did not undergo allo-SCT, including six who died before allo-SCT.
- Toxicity was higher in patients randomized to the high-dose cyclophosphamide arm and in those who received allo-SCT
- Exploratory landmark analyses did not show any association between either cranial irradiation (at 160 days) or allo-SCT (at 170 days) and outcome.
Conclusion
This study demonstrated that CNS involvement is significantly associated with poor outcomes in patients with newly diagnosed Philadelphia chromosome-negative ALL. This association was mainly due to excessive toxicity with no benefit from allo-SCT in this patient population. As this condition occurs in a minority of adult patients with ALL, it is unlikely that various treatment modalities will be tested in prospective controlled trials in patients with CNS involvement. Overall, these results can be a foundation for future studies on the allocation of CNS prophylactic measures in adult patients with newly diagnosed Philadelphia chromosome-negative ALL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


