All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Late relapse in ALL: Results from an 8-year follow-up of the MRC UKALLXII/ECOG E2993 trial
Few patients with acute lymphoblastic leukemia (ALL) are at risk of late relapse more than 3 years after achieving complete remission (CR). In one of the largest prospective trials in ALL, the Medical Research Council (MRC) in the United Kingdom and the Eastern Cooperative Oncology Group (ECOG) in the United States accrued 2,109 patients aged 15–60 in the MRC UKALLXII/ECOG E2993 trial and reported the outcomes of 609 patients with recurrent ALL after a median follow-up of 4.08 years.1
Chezi Ganzel et al. conducted a retrospective analysis in this study population with a longer follow-up of 8 years, to investigate the frequency and characteristics of late relapses, and recently reported their results in the British Journal of Haematology.2
Methods
- Retrospective analysis of the international MRC UKALLXII/ECOG E2993 trial.2
- Patients were < 55 years who
- underwent allogeneic hematopoietic stem cell transplant (HSCT) with a sibling or a matched unrelated donor,
- received autologous HSCT, or
- were administered long-term standard consolidation/maintenance therapy without transplant.
- Imatinib, a tyrosine kinase inhibitor, was given to a cohort of patients with Philadelphia chromosome (Ph)+ ALL, and 8-year follow-up results from this patient population are also presented in this summary.
- Early relapse was defined as relapse within 3 years of CR; late relapse was defined as first relapse after 3 years of CR; very late relapse was defined as relapse after 5 years of CR.
- Patients were monitored after relapse, and data on transplantation status, consecutive relapses, and survival were collected through a median follow-up duration based on patients who were alive.
- Two cytogenetic risk categories were defined:
- High risk: t(9;22), t(4;11), t(8;14), HoTr (low hypodiploidy [30–39 chromosomes], near triploidy [60–78 chromosomes]), or complex karyotype (≥5 abnormalities).
- Standard risk: patients who did not meet the above criteria.
Results2
A total of 1,909 patients were included in the first analysis, most of whom had Ph– ALL (see Figure 1).
Figure 1. Philadelphia chromosome status2,*
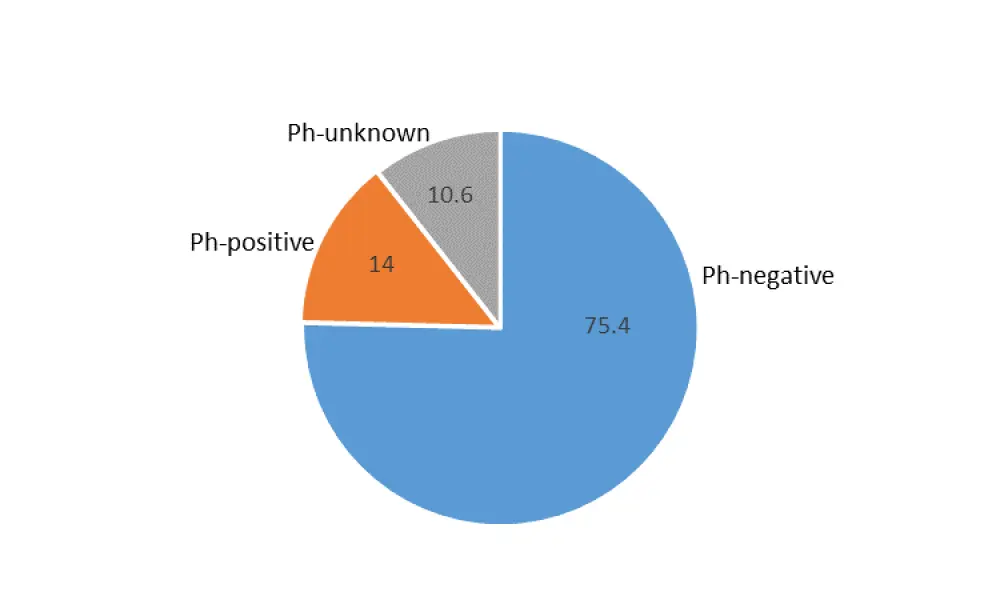
Ph, Philadelphia chromosome.
*Values given as %.
Overall, 92% of patients (n = 1,752) achieved CR status and were followed in this study. Characteristics of these patients are summarized in Table 1. After achieving CR, 711 (40.6%) patients received allogeneic HSCT, 206 (11.7%) had autologous transplant, and the remaining patients (nearly 50%) received chemotherapy only. The median follow-up duration in this patient population achieving CR was 96.5 months.
Among patients who achieved CR (n = 1,752), documented relapse was seen in 43.2% (n = 757; see Table 1).
- Early relapse rate was 91.3% (n = 691).
- Late relapse rate was 8.7% (n = 66).
- Very late relapse accounted for 2.8% of all relapses (n = 21 out of 66 late relapses).
Of the 732 patients who were alive at 3 years, 9% had late relapse. Of the 528 patients who were alive at 5 years, 4% had very late relapse. The late relapse rate among all patients achieving CR was 3.8%.
The median time to early and late relapses was 9 and 47 months (range 37–144), respectively. The cumulative risk of relapse after 3, 5, and 10 years was 40%, 43%, and 45%, respectively, while the cumulative risk of death without relapse was 17%, 18%, and 20%, respectively.
Following HSCT after first CR, the early relapse rate was higher than late relapse rate, and allogeneic HSCT was associated with a lower overall relapse rate. The early relapse rate was highest in patients who only received chemotherapy (see Table 1).
The median age for both early and late relapse was 32 years. The characteristics of patients achieving CR is provided alongside those experiencing early and late relapse in Table 1.
Table 1. Baseline characteristics of patients with early and late relapse among patients who achieved CR (n = 1,752)2
|
ALL, acute lymphoblastic leukemia; B-ALL, B-cell ALL; CR, complete response; HoTr, low hypodiploidy [30–39 chromosomes], near triploidy [60–78 chromosomes]; HSCT, hematopoietic stem cell transplant; T-ALL, T-cell ALL; WBC, white blood cell Highest rate among early and late responses are indicated in bold |
|||
|
Characteristic |
Patients who achieved CR (n = 1, 752) |
Early relapse (n = 691) |
Late relapse (n = 66) |
|---|---|---|---|
|
Lineage, % |
|
|
|
|
B-ALL T-ALL |
77.1 19.6 |
79.2 17.1 |
87.9 10.6 |
|
Median WBC count, × 109/L |
13 |
18 |
6 |
|
B-ALL T-ALL |
10 38 |
15 40 |
6 15 |
|
Cytogenetic risk category, n (%) |
|
|
|
|
Standard risk High risk Risk unknown |
810 (46.2) 400 (22.8) 542 (30.9) |
287 (35.4) 202 (50.5) 202 (37.3) |
38 (4.7) 5 (1.25) 23 (4.2) |
|
Median time to CR, days |
30 |
31 |
34 |
|
HSCT at CR, n (%) |
|
|
|
|
Autologous Allogeneic No HSCT |
206 (11.7) 711 (40.6) 835 (47.6) |
89 (43.2) 94 (13.2) 508 (60.8) |
7 (3.4) 9 (1.3) 50 (5.9) |
|
Cytogenetic abnormalities, n (%) |
|
|
|
|
t(1;19) Hyperdiploidy t(8;14) HoTr Complex t(4;11) |
31 (1.8) 132 (7.5) 18 (1) 34 (1.9) 56 (3.2) 78 (4.5) |
10 (32) 37 (28) 10 (55.6) 15 (44.1) 28 (50) 29 (37.2) |
0 5 (3.8) 0 2 (5.9) 1 (1.8) 0 |
Of 210 patients with BCR/ABL-negative ALL, 55 (26.2%) had Ph-like phenotype. 30 patients with Ph-like ALL experienced early relapse, and three had a late relapse.
In the second analysis, among 175 patients with Ph+ ALL who received imatinib treatment, most (90.9%, n = 159) achieved CR. Late relapse was recorded in two patients, while 63 relapsed early. The hazard ratio of relapse-free survival among patients who received imatinib during the induction period and those who received it at a later stage was not statistically significant (HR, 0.80; 95% CI, 0.54–1.2; p = 0.26). The late relapse rates among patients with Ph+ ALL who were or were not treated with imatinib was similar (3% versus 1.6%), however overall relapse rate was lower in patients receiving imatinib (40.9% versus 56.4%).
- The median survival in patients who experienced late relapse was higher (11.3 months) compared to patients with early relapse (5.4 months).
- 5-year OS rate after late and early relapse was 20% and 5.8%, respectively, indicating late relapse was associated with better survival.
Conclusion
Late relapse (> 3 years of CR) is not uncommon in adult patients with Ph– ALL, and although survival following late relapse is longer, outcome is still poor. The rate of late relapse was similar in patients with standard-risk or high-risk disease, among patients who were alive 3 years after achieving CR. HSCT also has been identified as a factor that positively affects both early and late relapse. While the introduction of imatinib in the treatment of Ph+ ALL patients reduced overall relapse rates, it had little impact on late relapses. The concept of Ph-like ALL was still unknown during the conduct of this study and had no therapeutic consequences for patients. The retrospective analysis confirmed the high rate of early relapse in these patients and further analysis is currently underway. This study did not uncover the cause of late relapse, and future research may investigate the possibility that late relapse could be a form of secondary ALL. A study limitation was that patients were off-study after relapse and monitored for survival only.
Overall, both patient- and disease-related factors are thought to play a role in survival, and the study data highlight the need for close monitoring for up to 5 years or even longer in patients with ALL achieving a CR.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

