All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Mini-hyper-CVD plus inotuzumab ozogamicin ± blinatumomab in adult R/R Ph− B-ALL
Historically, adult patients with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL) have experienced poor outcomes with intensive chemotherapy; however, the introduction of antibodies targeting CD19, CD20, and CD22 and chimeric antigen receptor T-cell therapies in pre-B-cell ALL (B-ALL) has transformed the treatment landscape for adults with ALL. This had led to the Food and Drug Administration (FDA) approval of four salvage therapies; blinatumomab, a CD19 bi-specific T-cell engager; inotuzumab ozogamicin (InO), an antibody-drug conjugate targeting CD22; and two chimeric antigen receptors, tisagenlecleucel and brexucabtagene autoleucel.
The phase II results of low-intensity chemotherapy (mini-hyperfractionated-CVD) combined with InO ± blinatumomab in adult patients with newly diagnosed Philadelphia-chromosome (Ph) negative B-ALL have been previously reported on the ALL Hub. Here, we summarize an article published by Kantarjian et al.1 in Journal of Hematology & Oncology on the long-term impact of mini-hyper-CVD + InO ± blinatumomab in adult patients with R/R Ph- B-ALL.
Study design
This phase II study (NCT01371630) included patients with R/R Ph− ALL CD22 positive pre-B-ALL, an Eastern Cooperative Oncology Group Performance Status of ≥3, normal cardiac function (ejection fraction >50%), and adequate organ function (serum bilirubin ≤1.95 mg/dL and serum creatinine ≤2.0 mg/Dl).
Patients 1 to 67 received:
- Mini-hyper-CVD for ≤8 cycles, with InO for the first 4 cycles
- InO at a dose of 1.3–1.8 mg/m2 on Day 3 of Cycle 1 and 0.8–1.3 mg/m2 on Day 3 of Cycles 2–4
- Maintenance included POMP (6-mercaptopurine + vincristine + methotrexate + prednisone) chemotherapy for ≤3 years
Patients 68 and onwards were given an amended treatment regimen:
- InO was administered in fractionated lower doses for the first four cycles of mini‑hyper‑CVD (0.6 mg/m2 on Day 2 and 0.3 mg/m2 on Day 8 of Cycle 1; 0.3 mg/m2 on Day 2 and 8 of Cycles 2–4), followed by 4 cycles of blinatumomab.
- Maintenance included 12 cycles of POMP and four cycles of blinatumomab.
The primary endpoints were the overall response rate, defined by a complete remission (CR); CR with incomplete platelet recovery; CR with incomplete hematologic recovery; and overall survival rate. Secondary endpoints included safety, relapse-free survival (RFS), the rate of subsequent stem cell transplantation (SCT), and minimal residual disease (MRD) negativity. Figure 1 outlines the study design for the protocol pre- and post-amendment.
Figure 1. Study design in A patients 1–67 and B patient 68 onwards*
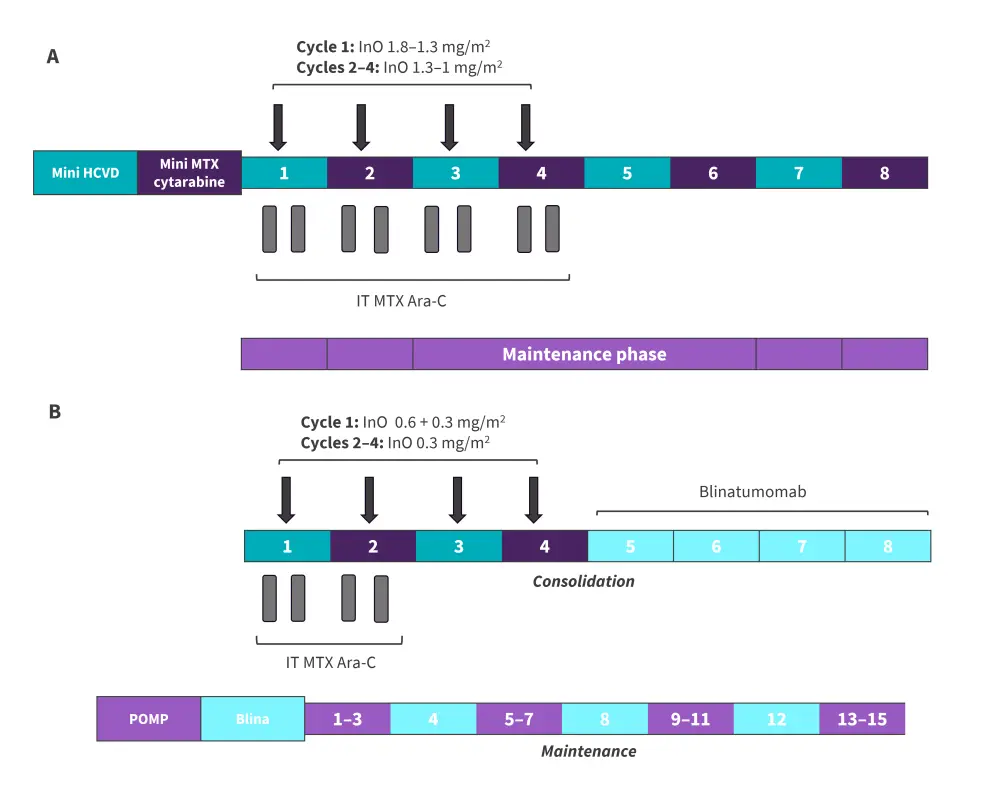
AraC, cytarabine; hyper-CVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone; blina, blinatumomab InO, inotuzumab ozogamicin; IT, intrathecal; MTX, methotrexate POMP, 6-mercaptopurine, vincristine, methotrexate, and prednisone.
*Data from Kantarjian, et al.1
Results
In total, 110 patients were treated with a median age of 37 years. Baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics*
|
ASCT, allogeneic stem cell transplantation; BM, bone marrow; CRLF2, colony receptor like factor 2; ECOG PS, Eastern Cooperative Oncology Group Performance Status; HeH, high hyperdiploidy; Ho-Tr, low hypodiploidy/near triploidy; IM, insufcient metaphases; ND, not determined; PB, peripheral blood; Ph, Philadelphia chromosome; Tt, tetraploidy; WBC, white blood cell. †High-risk cytogenetics include low hypodiploidy/near triploidy and KMT2A rearrangement ‡CLRF2 overexpression analyzed by fluorescent in situ hybridization or flow cytometry |
|||
|
Characteristic, % (unless otherwise stated) |
Overall |
Mini-hyper-CVD plus InO |
Mini-hyper-CVD plus InO + blinatumomab |
|---|---|---|---|
|
Median age (range), years |
37 (17–87) |
34 (17–87) |
42 (18–79) |
|
Sex |
|
|
|
|
Male |
47 |
46 |
49 |
|
ECOG PS of ≥2 |
17 |
16 |
19 |
|
WBC (× 109/L) |
|
|
|
|
Median (range) |
3.4 (0.1–194.7) |
3.7 (0.1–194.7) |
3.1 (0.8–129.9) |
|
≥50 |
4 |
3 |
5 |
|
Percentage of PB blasts (range) |
2.5 (0–97) |
3 (0–93) |
2 (0–97) |
|
Percentage of BM blasts (range) |
70 (6–98) |
72 (8–98) |
50 (6–96) |
|
BM blasts ≥50% |
63 |
70 |
51 |
|
Karyotype |
|
|
|
|
Diploid |
25 |
21 |
33 |
|
Other |
24 |
25 |
21 |
|
Complex |
12 |
15 |
7 |
|
KMT2A rearrangement |
9 |
12 |
5 |
|
Ho-tr |
11 |
6 |
19 |
|
HeH |
3 |
4 |
0 |
|
Tt |
2 |
1 |
2 |
|
IM/ND |
15 |
15 |
14 |
|
High-risk cytogenetics† |
20 |
18 |
23 |
|
Ph-like ALL |
18 |
10 |
30 |
|
CRLF2 overexpression‡ |
17 |
18 |
16 |
|
TP53 mutation |
32 |
38 |
28 |
|
Median CD22 expression (range) |
95.4 (14.3–100) |
95.6 (20–100) |
95.2 (14.3–99.9) |
|
Median CD19 expression (range) |
99.9 (0.5–100) |
99.9 (0.5–100) |
99.9 (10.5–100) |
|
≥20% CD20 expression |
25 |
18 |
37 |
|
Prior ASCT |
19 |
28 |
5 |
|
Salvage status |
|
|
|
|
Salvage 1 |
72 |
57 |
95 |
|
Salvage 2 |
15 |
22 |
5 |
|
Salvage 3 |
13 |
21 |
0 |
Updated efficacy1
The overall response rate was 83%, 80% of whom responded after the Cycle 1 and 20% responding after subsequent cycles. By salvage status, the overall response rate was 92%, 59%, and 57% after salvage 1, 2 and 3+, respectively. MRD negativity rates were higher after salvage 1 than salvage 2+. Morphologic responses and MRD-negativity rates by salvage status are reported in Figure 2.
Figure 2. Overall morphologic responses and B MRD-negativity by salvage status*
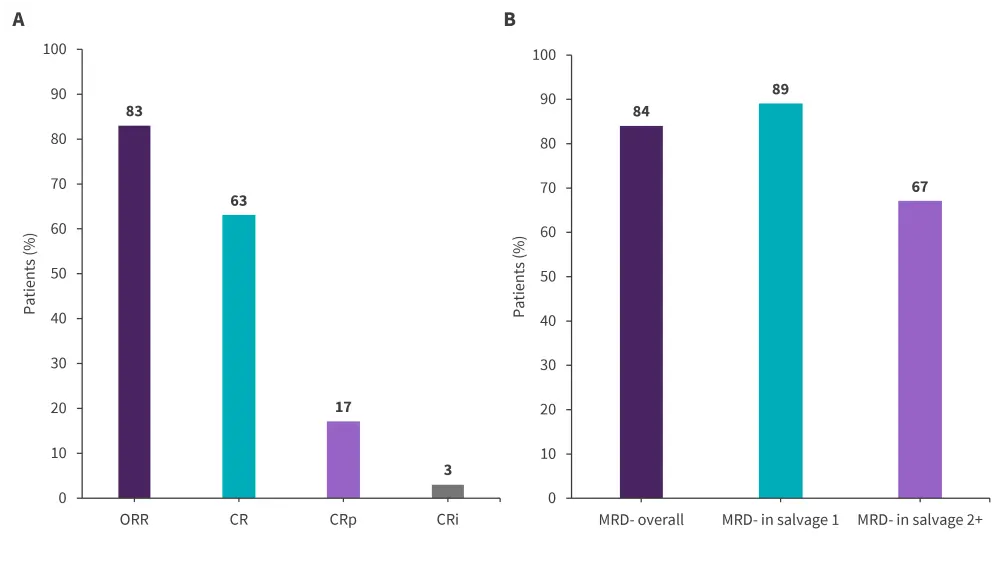
CR, complete remission; CRi, CR with incomplete count recovery; CRp, CR with incomplete platelet recovery; MRD, minimal residual disease; ORR, overall response rate.
*Data from Kantarjian et al.1
Of the 53 patients receiving SCT in CR, 29 occurred before the protocol amendment and 24 occurred after. For patients who did not undergo SCT, 10 were still alive at a median follow-up of 43 months.
At a median follow-up of 48 months, the estimated 3-year OS rate was 40% and the estimated RFS was 37%. The median OS and RFS was 17 months and 13 months, respectively. The 3-year OS rates significantly differed by salvage and MRD status and was similar in patients receiving and not receiving SCT (Figure 3). By TP53 mutational status (n = 60), the 3-year OS rate was 63% vs 9% for patients with wild-type TP53 versus mutated TP53, respectively.
Figure 3. 3-year OS by A treatment modality, B salvage line, C MRD status, and D SCT*†
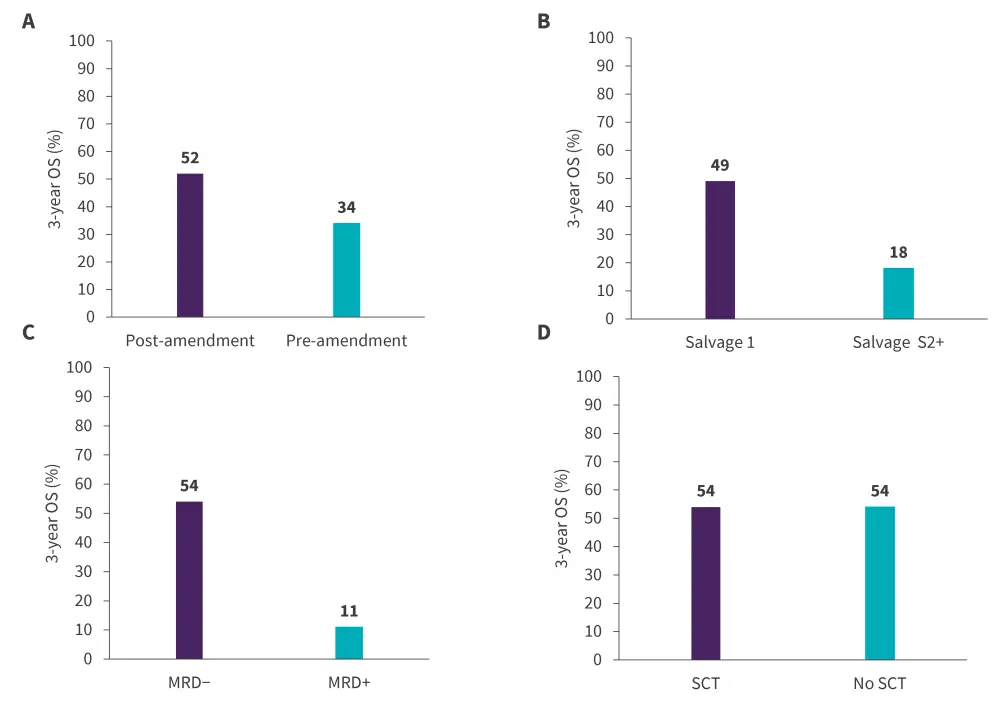
MRD, minimal residual disease; SCT, stem cell transplantation
*Data from Kantarjian, et al.1
†p = 0.0002 for difference in salvage line and p = 0.0005 for difference in MRD status
In a post-hoc analysis, this combination regimen yielded a higher median OS (17 months vs 6 months) and 3-year OS (40% vs 11%) when compared with InO monotherapy (p < 0.001). In the multivariate analyses, increased peripheral blood blasts percentage (p = 0.001), high-risk cytogenetics (p < 0.001), and presence of TP53 mutation (p = 0.004) at baseline were independently associated with worse survival outcomes. On the other hand, a lower fractionated dose of InO followed by blinatumomab was independently associated with improved survival (p = 0.033).
Safety
Overall, the treatment was well-tolerated with mostly Grade 1–2 adverse events (AEs) reported.
- A total of seven deaths were reported within the first 4 weeks of treatment before the protocol amendment; these were due to infections (n = 4), intracranial hemorrhage (n = 1), sinusoidal obstruction syndrome (n = 1), and unknown causes (n = 1).
- Nine deaths were reported in CR; these were due to infections (n = 2), myocardial infarction (n = 1), broncho pulmonary hemorrhage (n = 1), liver graft-versus-host disease (n = 1), and unknown causes (n = 4).
- The median time to platelet and neutrophil recovery in Cycle 1 was 23 days and 16 days, respectively; for subsequent cycles, the median time was 22 days and 17 days, respectively.
- There were no blinatumomab discontinuations due to treatment-related AEs.
Grade 3–5 AEs occurring in two or more patients are summarized in Table 2.
Table 2. Grade 3–5 AEs*
|
AE, adverse event. |
|||
|
AEs, % |
Grade 3 |
Grade 4 |
Grade 5 |
|---|---|---|---|
|
Infections, related and unrelated |
45 |
15 |
6 |
|
Hyperglycemia |
19 |
4 |
0 |
|
Pain† |
17 |
0 |
0 |
|
Hemorrhage |
13 |
1 |
2 |
|
Increased liver function tests |
12 |
2 |
0 |
|
Hypokalemia |
10 |
4 |
0 |
|
Increased bilirubin |
9 |
2 |
0 |
|
Cardiac |
9 |
0 |
1 |
Conclusion
In this longer-term follow-up analysis, mini-hyper-CVD plus InO ± blinatumomab was found to be effective and well-tolerated in adult patients with Ph− R/R ALL, with higher survival in those treated with vs without blinatumomab in Salvage 1, as well as those achieving MRD-negativity. This study yielded better survival outcomes when compared with historical data on single-agent InO or blinatumomab. Ongoing trials are currently investigating the value of MRD by next-generation sequencing in subsequent CR and the incorporation of dose-dense blinatumomab with mini-hyper-CVD plus InO. Future research investigating this combination treatment in multi-institutional trials is needed to establish this regimen as the new standard of care in R/R ALL.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



