All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Nelarabine for the treatment of T-cell acute lymphoblastic leukemia/lymphoma
Question 1 / 1
In the review by Miller et al., a phase III COG AALL0434 trial demonstrated promising outcomes with nelarabine for the treatment of T-ALL. What was the 5-year disease-free survival rate observed with nelarabine combined with C-MTX (escalating-dose methotrexate without leucovorin rescue + pegaspargase)?
A
78%
B
83%
C
89%
D
91%
In March 2023, nelarabine was approved by the U.S. Food and Drug Administration (FDA) for the treatment of T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL). Here, we summarize a review of nelarabine, published by Miller et al. in Therapeutics and Clinical Risk Management,1 that highlights the potential challenges and future directions of nelarabine in the treatment of T-ALL/LBL.
Nelarabine clinical trials
Nelarabine, a guanine nucleoside analog, has been evaluated in numerous clinical trials; eight are currently ongoing (Figure 1).
Figure 1. Active clinical trials evaluating nelarabine*
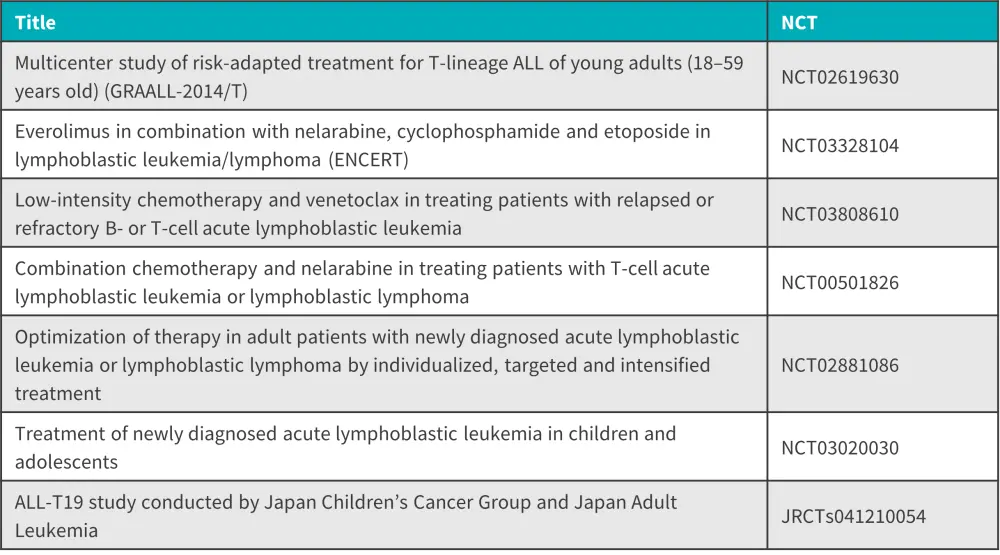
ALL, acute lymphoblastic leukemia.
*Adapted from Miller, et al.1
The ALL Hub has previously published key updates from the European Hematology Association (EHA) 2023 Congress, including some of the trials mentioned above:
- Novel treatment in Philadelphia negative ALL: Key updates from EHA 2023 (NCT03808610)
- Novel treatment approaches in Ph+ ALL: Key updates from EHA 2023 (NCT02881086)
Results from nelarabine trials
Nelarabine has shown positive results in clinical trials for both children and adults as a single-agent or in combination.
Phase I trial
- Of the 28 patients with refractory T-ALL treated with compound 506U78 (5–75 mg/kg), 14 patients achieved a complete response and nine patients achieved a partial response.
- Dose-limiting neurotoxicity, including weakness, ataxia, confusion, and coma, were observed in some patients at the 40 mg/kg and 60 mg/kg dose level; however, no neurotoxicity was observed in children treated with 40 mg/kg dose.
Phase II trial
- The Children’s Oncology Group (COG) trial (P9673) included 153 children and young adults with relapsed T-ALL or progressive disease. Nearly half of the first relapse cohort entered second remission with single-agent therapy after receiving an intravenous dose of 650 mg/m2 on Days 1–5.
- In another trial, similar results were seen in adults with relapsed T-ALL with a weekly intravenous dose of 1.5 gm/m2 on Days 1, 3, and 5.
- Adverse events of nelarabine included nausea, vomiting, myeloid suppression, and chemotherapy-induced immunosuppression.
- In some patients, significant peripheral and central neuropathies, and more rarely, rhabdomyolysis was also observed.
- A phase II study (AALL00P2) incorporated nelarabine into a COG-Berlin-Frankfurt-Munich backbone:
- Patients who received nelarabine showed improved event-free and overall survival, with manageable adverse events in a multi-agent setting.
- A phase II trial by The Japan Children’s Cancer Group and Japan Adult Leukemia Study Group incorporating nelarabine onto AIEOP-BFM (Associazione Italiana di Ematologia Oncologia Pediatrica-Berlin-Frankfurt-Munich) ALL 2000 backbone, with similar dosing of nelarabine to the previously mentioned COG trial:
- 3-year event-free survival of 86.4% (95% confidence interval [CI], 82.3–89.7%)
- 3-year overall survival of 91.3% (95% CI, 87.7–93.8)
- The trial also combined nelarabine with high-dose methotrexate (HD-MTX) which showed similar survival and safety outcomes.
Phase III trial
- In a phase III study, 1,895 patients with T-ALL or T-LBL aged 1–31 years received either Capizzi-style methotrexate vs HD-MTX in the interim maintenance phase and/or six 5-day courses of nelarabine.
- Adverse events were observed as expected but not as severe as seen in highly pretreated patients enrolled in earlier trials.
- Two of 43 patients experienced post-induction Grade 5 neurological events; however, event-free survival and overall survival for this subset of patients was approximately 50%, significantly higher than prior therapeutic approaches that did not include nelarabine.
Improving survival in patients with high-risk features
- In the AALL0434 study, a 5-year disease-free survival of 91% (n = 147) was observed in the Capizzi-style methotrexate with nelarabine treatment arm.
- Fewer isolated central nervous system (CNS), combined CNS, and bone marrow relapses were observed with nelarabine.
- In addition, disease-free survival for patients with CNS-3 T-ALL treated with HD-MTX and nelarabine at 4 years was 93.1 ±5.2%, compared with a 4-year disease-free survival of 70.2 ±5.8% in patients treated with HD-MTX without nelarabine.
Relapse strategies
Treatment in children and young adults with relapsed T-ALL and T-LBL is challenging, and the reinduction treatment plan depends on the patient’s previous exposure to nelarabine. Other agents that have demonstrated promising efficacy as a single agent or in combination in heavily pretreated patients with relapsed disease include:
- daratumumab
- alemtuzumab
- venetoclax
- navitoclax
- bortezomib
- palbociclib
- ribociclib
- dasatinib
- ruxolitinib
Many of these additional agents are not yet approved by the U.S. FDA for relapsed/refractory childhood T-ALL/T-LBL; however, novel combinations should be considered with caution and shared decision-making. If the safety profile is thought to be tolerable, the combination of any of these agents with nelarabine should be considered regardless of previous nelarabine exposure. Chimeric antigen receptor T-cell therapy is also a potential option.
Conclusion
Overall, the review demonstrates the safety and efficacy of nelarabine in the treatment of T-ALL and T-LBL. It highlights the role of nelarabine in treating refractory disease and patients with CNS-3 disease. Caution must be taken to ensure safety in patients with new, relapsed, or refractory diseases and minimize adverse events. Nelarabine has the potential to become an essential component of future treatment regimens with careful consideration of timing, dosing, and potential adverse events.1
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

