All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Do you know... Which of the following treatment options would you select for a patient with a high disease burden of relapsed/refractory Philadelphia chromosome-negative B-cell acute lymphoblastic leukemia?
The treatment landscape for patients with Philadelphia chromosome negative (Ph−) acute lymphoblastic leukemia (ALL) has expanded with the introduction of targeted therapies; therefore, combining these agents with chemotherapy is a key treatment of interest. Below, we summarize three key presentations on novel therapies for the treatment of Ph− ALL, presented at the European Hematology Association (EHA) 2023 Congress.
Haddad presented overall survival (OS) and relapse-free survival (RFS) data for venetoclax in combination with mini-hyper-cyclophosphamide, vincristine, and dexamethasone (CVD) in relapsed/refractory (R/R) Ph− ALL, and a study of inotuzumab ozogamicin (InO) with or without blinatumomab in older patients with newly diagnosed Ph- ALL1,2; Short presented response rates of hyper-CVAD (cyclophosphamide, vincristine doxorubicin, and dexamethasone) with blinatumomab and InO in de novo Ph− ALL.3
Mini-hyper-CVD with venetoclax in R/R Ph− ALL1
This open-label, phase II trial (NCT03808610) investigated the combination of venetoclax with low intensity mini-hyper-CVD in patients with R/R Ph− B-cell or T-cell ALL (B-ALL; T-ALL). The study endpoints included RFS and OS, other endpoints included complete response (CR), overall response rate (ORR), and median time to platelet and neutrophil recovery.
- Overall, 23 patients were enrolled with a median age of 45 years, 52% were male, 78% had B-cell ALL and 22% had T-cell ALL; the median number of prior treatments was two and 57% of the patients had prior allogeneic stem cell transplant.
- Median 1-year OS and RFS was 8.1 months and 6.4 months, respectively, with a 1-year RFS rate of 15% and OS rate of 37%; survival was worse in patients with adverse cytogenetics compared with those without (median OS, 6 months vs 12 months).
- In total, 55% of patients responded to therapy and 41% achieved a CR (n = 9); ORR was 67% among patients who underwent a bone marrow transplant after Cycle 1 (n = 18); median time to platelet and neutrophil recovery was 27 days and 21 days, respectively, after Cycle 1.
- Mortality rates were 0% and 13% after 30 days and 60 days, respectively.
Presenter’s conclusions
Mini-hyper CVD in combination with venetoclax was effective with a favorable safety profile in patients with R/R Ph− B-cell or T-cell ALL.
Mini-hyper-CVD-InO with or without blinatumomab in newly diagnosed Ph− ALL3
Haddad presented another open-label, phase II trial (NCT01371630) investigating InO plus mini-hyper-CVD in combination with blinatumomab in newly diagnosed patients aged ≥60 years with Ph− pre-B ALL. The study endpoints were the duration of complete remission and OS. Other endpoints included CR, ORR, and minimal residual disease (MRD) negativity rate.1
- The study included 83 patients with a median age of 68 years, 34% were ≥70 years old, 13% had an Eastern Cooperative Oncology Group Performance Status of ≥2, 39% had a TP53 mutation, and 5% had central nervous system disease at diagnosis.
- ORR was 99%, of which 90% achieved CR. The MRD negativity rate was 79% at Cycle 1 and 94% overall.
- Five-year duration of complete remission was 78% and OS was 48%; patients aged 60–69 without poor-risk cytogenetics had better survival than patients aged ≥70 years (72% vs 38%).
- Three patients developed veno-occlusive disease (VOD), two after subsequent stem cell transplantation.
Presenter’s conclusions
Mini-hyper-CVD plus InO plus blinatumomab was effective with a tolerable safety profile in this subgroup of older patients with newly diagnosed Ph− ALL.1 Patients aged 60–69 years without poor-risk cytogenetics and had significantly improved 5-year OS (72%).
Hyper-CVAD with blinatumomab and InO in newly diagnosed Ph- ALL2
Short presented an open-label, phase II study (NCT02877303) examining the efficacy and safety of hyper-CVAD in combination with sequential blinatumomab with or without InO in patients with newly diagnosed Ph− B-cell ALL. The study endpoints were CR, RFS, and OS. Other endpoints included flow MRD negativity rate and 30-day mortality.
- Overall, 72 patients were enrolled with a median age was 34 years, 85% had an Eastern Cooperative Oncology Group Performance Status of 0–1, 8% had central nervous system involvement at diagnosis, and 18% had a TP53 mutation.
- RFS at 1-year in the hyper-CVAD + blinatumomab + InO and hyper-CVAD + blinatumomab groups was 97% and 82%, respectively (p = 0.07).
- OS at 1 year in the hyper-CVAD + blinatumomab + InO and hyper-CVAD + blinatumomab groups was 100% and 87%, respectively (p = 0.04).
- Response rates were higher in patients treated with InO and blinatumomab compared with blinatumomab alone, with CR after induction and at any time achieved by 84% and 100%; 69% were flow MRD negative after induction and 95% were at any time (Figure 1).
- There were no cases of mortality at Day 30 posttreatment and no cases of VOD/sinusoidal obstruction syndrome or discontinuations were observed in patients treated with InO; one patient discontinued blinatumomab due to a Grade 2 neurotoxicity event.
Figure 1. Response rates*
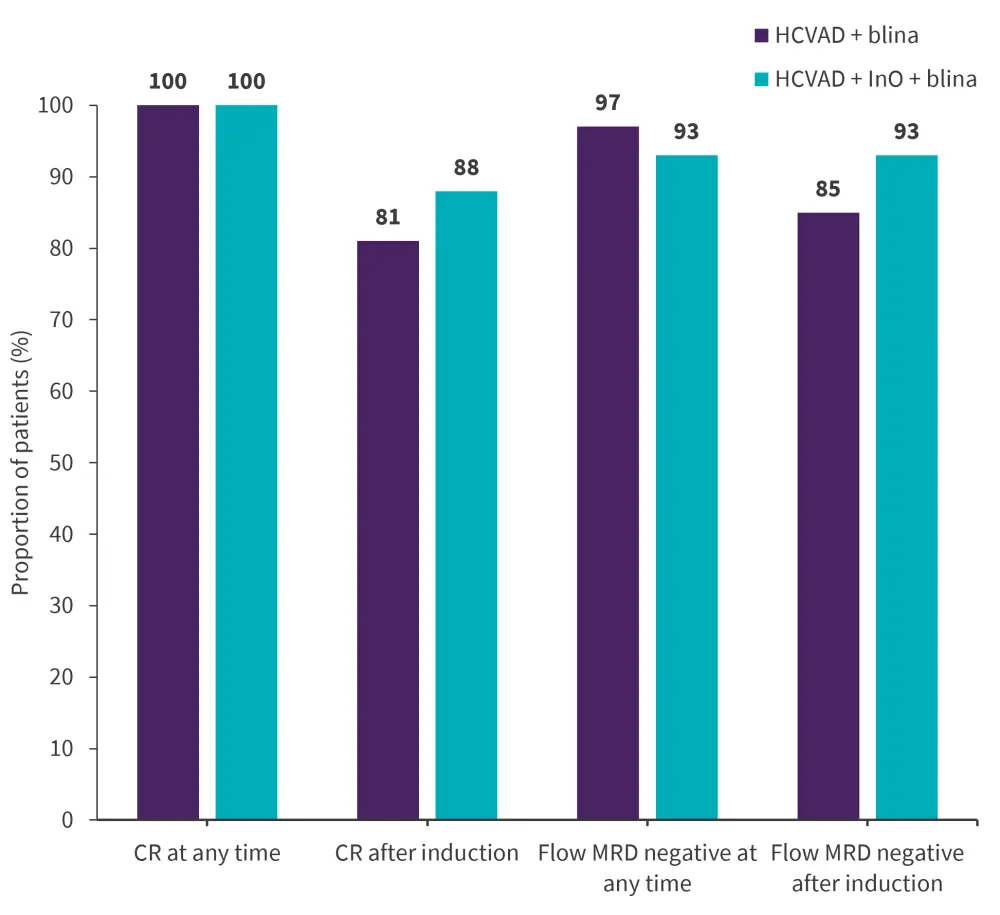
CR, complete response; blina, blinatumomab; HCVAD, hyper-cyclophosphamide, vincristine doxorubicin, and dexamethasone; InO, inotuzumab ozogamicin; MRD, minimal residual disease.
Data from Short.3
Presenter’s conclusions
Hyper-CVAD combined with sequential blinatumomab ± InO is an effective first-line therapy for patients with newly diagnosed Ph− B-cell ALL.3 The addition of InO improves response and safety outcomes compared with blinatumomab alone.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Nicholas J. Short
Nicholas J. Short