All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Educational theme | Treatment of Ph-like ALL
Do you know... Which of the following therapies is FDA approved for the treatment of Philadelphia-like acute lymphoblastic leukemia?
Our previous educational theme articles have focused on the biology, genomic landscape, epidemiology, and prognosis of Philadelphia (Ph)-like acute lymphoblastic leukemia (ALL) and the diagnosis of Ph-like ALL. This educational theme article discusses treatment strategies for Ph-like ALL, summarized from several recent articles published by Harvey and Tasian in Blood Advances in 2020,1 Prescott, et al., in Current Hematologic Malignancy Report in 2020,2 Aldos and Advani in Best Practice & Research Clinical Haematology in 2021,3 Vettenranta, et al., in Frontiers in Pediatrics in 2022,4 Zhao, et al., in Blood in 2020,5 and Tran and Tasian in Best Practice & Research Clinical Haematology in 2021.6
Ph-like ALL is a high-risk subset of B-cell precursor ALL (B-ALL), associated with poor treatment response rates and high relapse rates. Ph-like ALL is most common in the adolescent and young adult (AYA) population, although it also occurs in pediatric and older adult populations.2
In general, conventional chemotherapy regimens do not yield a positive response in patients with Ph-like ALL, due to high rates of induction failure, post-induction minimal residual disease (MRD) positivity, relapse, and low survival rates compared with non-Ph-like ALL. Studies have also demonstrated worse overall survival and event-free survival (EFS) compared with other types of B-ALL, with the exception of Ph-positive ALL.2,3 Moreover, compared to patients with Ph-positive ALL, patients with Ph-like ALL have inferior survival outcomes across the age spectrum.6
- A lower EFS rate was reported in children with National Cancer Institute (NC) classified high-risk Ph-like B-ALL compared with non-Ph-like ALL (63% vs 86%; p < 0.0001); this was true for all patients with Ph-like ALL irrespective of randomization (COG AALL0232 trial; NCT00075725).
- Statistically inferior outcomes were reported in children with NCI standard risk (SR) Ph-like ALL compared with those who had SR non-Ph-like ALL (7-year EFS, 82.4% vs 90.7%; p = 0.002) in the COG AALL0331 trial (NCT00103285).
- Unfavorable outcomes were seen in the AYA population with Ph-like ALL compared with non-Ph-like ALL (3-year EFS, 42% vs 69%; p = 0.008) in the CALBG 10403 trial (NCT00558519).
- Another study reported worsening of outcomes with increasing age with a 5-year EFS rate of 40.4%, 29.8%, and 18.9% for young adults (range, 21–39 years of age), adults (range, 40–59 years of age), and older adults (range, 60–86 years of age), respectively.
Treatment strategies
To date, there is no standard treatment guideline for patients with Ph-like ALL.3 Outcomes for frontline therapy in adults with Ph-like ALL have been poor for most treatment protocols, including modern pediatric-inspired regimes. Confirmation of a Ph-like ALL diagnosis is essential to enable earlier therapeutic intervention and improve outcomes.2
Treatment typically starts with induction chemotherapy, which may change as definitive diagnostic results are returned; however, genetic testing can take weeks to complete and may have no impact on the choice of therapy. Many treatment centers will also recommend enrolment in appropriate clinical trials, dependent upon patient eligibility; this is particularly true for older patients, as their outcomes with conventional chemotherapy are poor.3 As Ph-like ALL is often chemorefractory, treatment centers may place emphasis on mitigating treatment-related toxicities to allow for the early introduction of salvage therapy with novel agents.3
It is uncertain whether early MRD clearance in adult patients with Ph-like ALL is beneficial, as is the case for other B-ALL subtypes. This suggests post-induction therapy should be intensified irrespective of early MRD response status, which could be achieved by the escalation of post-induction chemotherapy or consolidation with allogeneic hematopoietic stem cell transplantation (AlloHSCT). However, this carries the risk of excessive toxicity, particularly in older adults.3
Tyrosine kinase inhibitors
As most cases of Ph-like ALL involve genetic alterations in kinases and/or cytokine receptors, the application of tyrosine kinase inhibitors (TKIs) has become an area of active research. In Ph-like ALL associated with ABL-class fusions and JAK-STAT activating alterations, TKIs have shown promising outcomes in preclinical trials. These encouraging results have led to a number of further clinical trials examining these novel agents in combination with established chemotherapy regimens. In addition, numerous single case reports exist detailing the benefits of TKIs dasatinib and imatinib when used to treat Ph-like ALL with ABL1-class fusions, as well as for ruxolitinib.3
Patients who had Ph-like ALL, various kinase fusions, and were treated with a pediatric-derived and MRD stratified protocol, showed a significantly lower complete response rate (74.1% vs 91.5%; p = 0.044) and EFS (33.5% vs 66.2%; p = 0.005) compared with non-Ph-like patients in the GIMEMA LAL2116 D-ALBA trial; this has been previously reported on the ALL Hub. Significant differences were observed among the various genomic subgroups of Ph-like ALL, with JAK2/EPOR-rearranged and CRLF2-rearranged/JAK2 mutant cases showing a 5-year EFS of 26.1% and 38.8%, respectively. However, cases harboring other JAK/STAT and RAS pathway mutations showed more favorable EFS of 68.3% and 85.7%, respectively (p < 0.0001). Based on the specific fusion subtype, there were differences in MRD response and clinical outcomes in patients with ABL-class fusions. Interestingly, children with PDGFRB and ABL2 fusions appear to have inferior EFS and MRD levels >10-2 at the end of induction compared with those who had ABL1 or uncommon CSF1R rearrangements.6
Targeted therapies
The chemotherapy-resistant nature of Ph-like ALL has led to interest in the use of targeted immunotherapies, such as those already approved for the treatment of B-ALL, as a novel treatment strategy. Blinatumomab, a bispecific CD3/CD19 antibody, currently approved by the U.S. Food and Drug Administration (FDA) for the treatment of B-cell precursor ALL, demonstrated an overall response rate of 55% and a high response rate in those with CRLF2-rearranged Ph-like ALL (75%).3,5 A number of different drugs and strategies are currently under investigation, including inotuzumab ozogamicin and forms of chimeric antigen receptor T-cell therapy.3
Combination therapies
Advances in sequencing technologies have enabled the identification of a diverse range of kinase-activating translocations and mutations that may be amenable to therapeutic options in patients with Ph-like ALL.
Preclinical studies have demonstrated a synergistic effect when combining mTOR (target of rapamycin complex) inhibitors with JAK inhibitors in Ph-like ALL. There are several ongoing phase II/III clinical trials combining TKIs with chemotherapy to evaluate this approach in children and adults with Ph-like ALL (Figure 1).3
Figure 1. Key ongoing clinical trials focused on the treatment of Ph-like ALL*
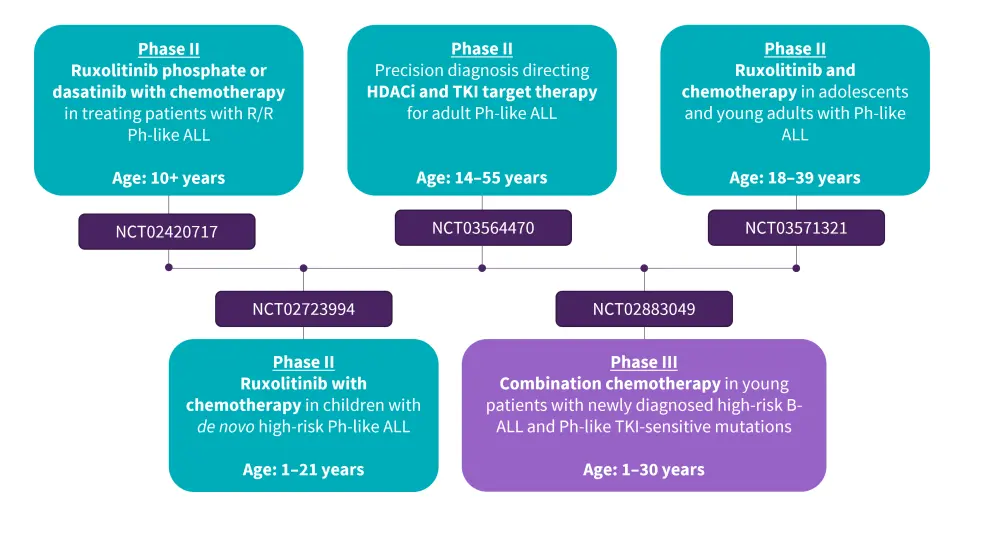
ALL, acute lymphoblastic leukemia; B-ALL, B-cell ALL; HDACi, histone deacetylase inhibitors; Ph, Philadelphia; R/R, relapsed/refractory; TKI, tyrosine kinase inhibitor.
*Adapted from Aldoss and Advani.3
Hematopoietic stem cell transplant
AlloHSCT is a well-established means of preventing relapse and is recommended as consolidation therapy for adults with high-risk ALL. An MRD-oriented approach was commenced in the GIMEMA LAL1913 study, demonstrating that more patients with Ph-like ALL were allocated to transplant compared to patients with non-Ph-like ALL (53% vs 20%, respectively).3 AlloHSCT is suggested for adults with Ph-like ALL, who have persistent MRD post-consolidation, on an individual basis (considering age, chemotherapy resistance, and other risk factors). For older patients, transplant is considered as consolidation therapy based on donor availability, irrespective of MRD response.3 Although HSCT has shown promise in patients with Ph-positive ALL, its role in the treatment of Ph-like ALL is still unclear. Further studies are needed to fully elucidate the value of HSCT in this disease subset and it may yet contribute to a total immune therapy in the future.4
Conclusion
The treatment of Ph-like ALL has improved significantly over the last decade. While there are still many clinical challenges in this area, new advances such as the use of TKI’s, immunotherapies, and chimeric antigen receptor T-cell therapies are offering hope to patients and healthcare providers. Due to the high occurrence of kinase-activating mutations, there is a possibility that unique approaches in precision medicine such as combination therapies could lead to improved treatment options. The variability and small number of patients with Ph-like ALL make it difficult to plan clinical trials and develop standardized treatment plans; however, progress is being made in this area.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content






