All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Optimizing treatment for adults with R/R Ph-negative B-cell ALL
Featured:
Do you know... What is the optimal initial dose of inotuzumab ozogamicin when used as a bridging therapy to CAR T-cell therapy?
During the ALL Hub Steering Committee meeting, Wendy Stock chaired a discussion on optimizing treatment for adults with relapsed/refractory (R/R) Philadelphia chromosome-negative B-cell acute lymphoblastic leukemia (B-ALL), featuring André Baruchel, Nicolas Boissel, Mark Litzow, José María Ribera, Elias Jabbour, and Sabina Chiaretti.
Optimizing treatment for adults with R/R Ph-negative B-cell ALL
This discussion was based on a patient case (Figure 1) and focused on four key questions:
- What are the therapy options to induce a remission prior to chimeric antigen receptor (CAR) T-cell therapy?
- What is the optimal use of inotuzumab ozogamicin (InO) to induce a remission and reduce the disease burden (dosing, number of cycles)?
- What should be monitored during therapy, and how can monitoring impact treatment to maximize patient outcomes?
- What are your management strategies following CAR T-cell therapy?
Figure 1. Patient case
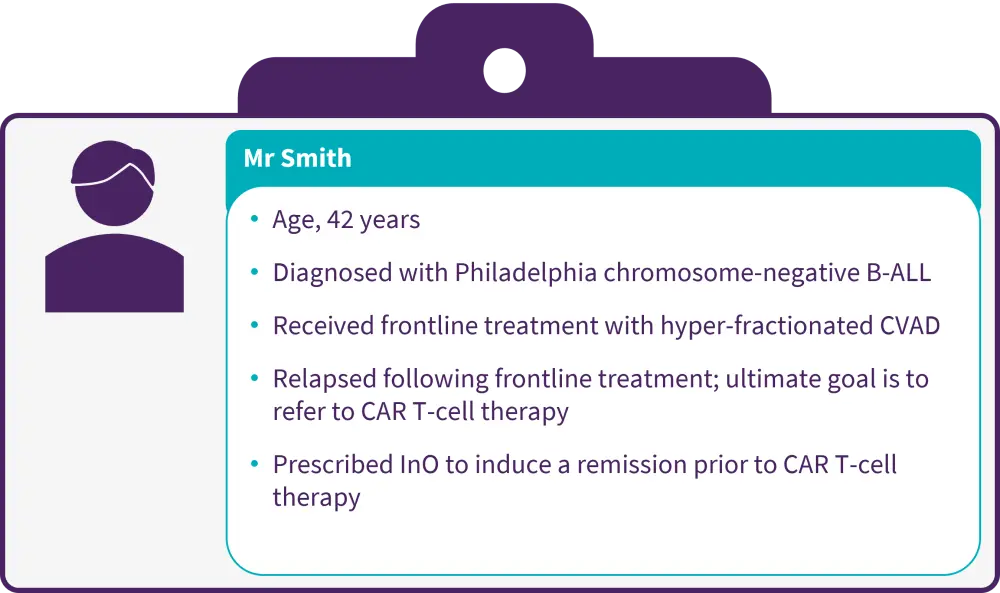
B-ALL, B-cell acute lymphoblastic leukemia; CAR, chimeric antigen receptor; CVAD, cyclophosphamide-vincristine-daunorubicin-dexamethasone; InO, inotuzumab ozogamicin.
Stock presented results from an analysis of the impact of bridging therapy with and without InO in adult patients with R/R B-ALL prior to treatment with obecabtagene autoleucel in the phase Ib/II FELIX trial (NCT04404660). Bridging therapies containing InO effectively reduced disease burden and, although the patients in the InO group had a higher baseline bone marrow blast percentage (bridging with InO, 73%; bridging without InO, 34%; no bridging therapy, 20%), efficacy outcomes were better in those that received bridging with vs without InO (Table 1).1
Table 1. Efficacy outcomes following obe-cel infusion following bridging therapy with and without InO in the FELIX trial*
|
Efficacy outcomes |
Bridging with InO |
Bridging without InO |
No bridging |
|
CR/CRi, % |
83 |
75 |
100 |
|
DOR, months |
21.2 |
14.1 |
NE |
|
Median EFS, months |
22.1 |
9.0 |
NE |
|
18-month EFS probability, % |
56 |
38 |
75 |
|
Median OS, months |
23.8 |
14.1 |
NE |
|
CR, complete remission; CRi, CR with incomplete hematologic recovery; DOR, duration of response; EFS, event-free survival; InO, inotuzumab ozogamicin; NE, not evaluable; obe-cel, obecabtagene autoleucel; OS, overall survival. |
|||
Stock then highlighted the key considerations for the use of InO in patients with R/R B-ALL, including advantages and limitations, potential dose modifications when used as a bridging therapy, toxicities associated with InO, and the impact of measurable residual disease (MRD) on outcomes. Finally, Stock discussed alternative bridging strategies, and potential complications following CAR T-cell therapy.
Discussion
Key discussion points included:
- The goal of bridging therapy is cytoreduction with minimal toxicity, and achieving a complete remission may not be necessary.
- One cycle of InO with standard dosing (0.8 mg/m2 on Day 1 and 0.5 mg/m2 on Days 8 and 15) prior to CAR T-cell therapy may be sufficient, and MRD monitoring can help guide treatment decisions.
- Treatment with InO can result in rapid responses irrespective of baseline disease burden; however, as with all treatments, disease biology can affect the efficacy.
- B-cell aplasia and MRD should be monitored often to assess treatment response, and monitoring strategies can change depending on the genetics of the disease and the type of CAR T-cell therapy used.
- The management of CAR T-cell therapy complications is improving, and earlier intervention may improve outcomes.
- The panel discussed whether patients should proceed to transplantation following CAR T-cell therapy, and the factors impacting this decision.
- There is a need to evaluate whether targeted immunotherapy bridging alone may suffice to proceed directly to transplantation.
Listen to this discussion as a podcast:
Optimizing treatment for adults with R/R Ph-negative B-cell ALL
Your opinion matters
I commit to reviewing the latest clinical data and recommendations for the optimal use of inotuzumab ozogamicin in patients with R/R B-ALL.
This educational resource is independently supported by Pfizer. All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 José María Ribera
José María Ribera Wendy Stock
Wendy Stock Sabina Chiaretti
Sabina Chiaretti Mark Litzow
Mark Litzow Elias Jabbour
Elias Jabbour André Baruchel
André Baruchel Nicolas Boissel
Nicolas Boissel

