All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Phase III study confirms superior outcomes using haploidentical donors in patients with pretransplant MRD-positive ALL
The criteria for choosing the most appropriate transplant donor is still a topic of hot debate. Haploidentical donor transplantation (HIDT) has been shown to yield comparable outcomes to matched sibling donor transplantation (MSDT) in the setting of acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and lymphomas, and may even be preferred over MSDT in some high-risk patients due to the better graft-versus-leukemia reaction experienced. However, most studies in this context have been retrospective in nature and may not serve as suitable evidence for treatment decisions. Therefore, selection of optimal donor options needs further investigation in prospective trials.
In this prospective, genetically randomized study (NCT02185261) recently published in Journal of Hematology & Oncology, Ying-Jun Chang and colleagues evaluated donor options in patients with ALL who were positive for measurable residual disease (MRD) pre-transplantation and underwent MSDT or HIDT.1
Study design
The inclusion criteria for this study were patients aged 3–65 years with ALL in complete remission (CR) and pre-hematopoietic stem cell transplantation (HSCT) MRD positivity. Exclusion criteria were severe cardiac, renal, or hepatic disease, a prior transplant, and hypersensitivity to rabbit anti-thymocyte globulin (ATG) if a haploidentical donor was available.
A total of 745 patients with ALL who achieved CR after chemotherapy treatment were included (Figure 1). Initially, 20 patients were excluded because they received HLA-matched unrelated donor transplant, and 517 patients were then excluded due to achieving CR with pre-transplantation MRD negativity. Finally, 208 patients with positive pre-transplantation MRD were genetically randomized to receive HIDT (169 patients) or MSDT (39 patients).
Figure 1. CONSORT (the Consolidated Standards of Reporting Trials) diagram1
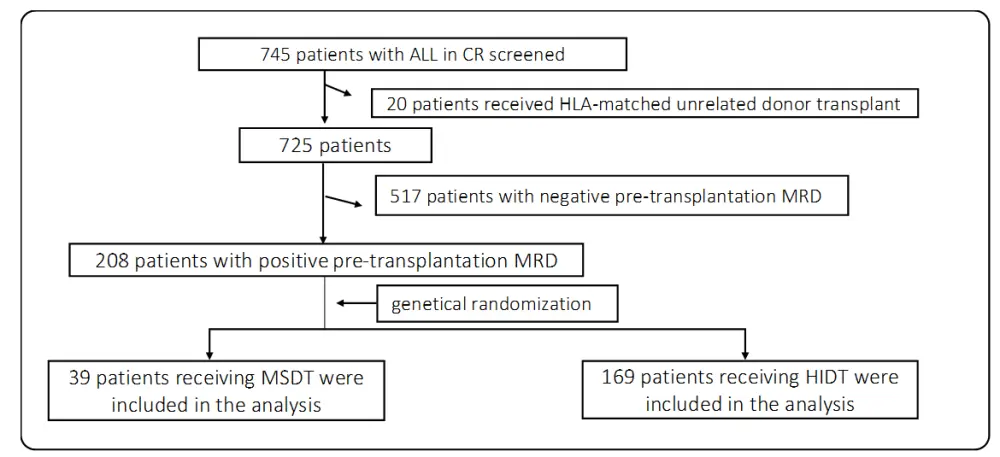
ALL, acute lymphoblastic leukemia; CR, complete remission; HIDT, haploidentical donor transplantation; HLA, human leukocyte antigen; MRD, measurable residual disease; MSDT, HLA-matched sibling donor transplantation.
Results
Patient and donor characteristics are summarized in Table 1. Transplant outcomes between patients who underwent HIDT and those who received MSDT are detailed in Table 2. Uni- and multivariate analysis of factors associated with transplantation outcomes are summarized in Table 3. Primary causes of death among patients that underwent allogeneic stem cell transplantation are given in Table 4.
The Epstein-Barr virus reactivation at Day 100 post-transplantation in the HIDT group (15%; 95% CI, 10–21) was significantly higher than that of the MSDT group (0%; p = 0.011).
Multivariate analysis demonstrated that: (Table 3)
- Higher than median CD34 cell count infused (when comparing less vs higher than median) was the only significant element associated with both neutrophil (HR, 0.749; 95% CI, 0.567–0.988; p = 0.041) and platelet engraftment (HR, 0.671; 95% CI, 0.506–0.889; p = 0.006).
- Higher levels of pretransplant MRD increased the risk of MRD positivity after transplantation (HR, 1.281; 95% CI, 1.043–1.572; p = 0.014).
- Transplant modality (HIDT vs MSDT) was also linked with MRD positivity after transplantation (HR, 0.492; 95% CI, 0.280–0.866; p = 0.018).
Similar to factors associated with overall survival (OS; Table 3), multivariate analysis also demonstrated a significant association with leukemia-free survival for:
- Pretransplant MRD levels (HR, 1.747; 95% CI, 1.011–3.021; p = 0.046),
- Transplant modality (HIDT vs MSDT; HR, 0.425; 95% CI, 0.252–0.718; p = 0.001),
- Disease status (≥ CR2 vs CR1; HR, 1.789; 95% CI, 1.059–3.024; p = 0.030),
- Grades II–IV acute graft-versus-host disease (GvHD) (HR, 1.727; 95% CI, 1.036–2.879; p = 0.036),
- Chronic GvHD (yes vs no; HR, 0.476; 95% CI, 0.282–0.803; p = 0.005), and
- Posttransplant MRD positivity (HR, 1.707; 95% CI, 1.059–2.752; p = 0.028).
This study conducted analysis for 128 patients with very low MRD levels (sensitivity at 0.01%) in the bone marrow for pre-HSCT. Of those 128 patients, 24 received MSDT and 104 had HIDT. Given as patients who underwent MSDT versus HIDT:
- 3-year cumulative incidence of relapse: 46% (95% CI, 24–68) vs 29% (95% CI, 20–38); p = 0.159.
- Leukemia-free survival: 37% (95% CI, 17– 57) vs 55% (95% CI, 45–65); p = 0.175.
- Overall survival: 43% (95% CI, 23–63) vs 59% (95% CI, 49–69); p = 0.270.
- Non-relapse mortality rates were comparable: 17% (95% CI, 0–34) vs 15% (95% CI, 8–22); p = 0.782.
The 517 patients who were pre-HSCT MRD negative were also analyzed for either MSDT (n = 92) or HIDT (n = 425). Compared to patients receiving HIDT, patients who underwent MSDT had similar outcomes for
- 3-year cumulative incidence of relapse (16% vs 15%; p = 0.776),
- leukemia-free survival (72% vs 68%; p = 0.463),
- OS (73% vs 70%; p = 0.528),
- non-relapse mortality (12% vs 16%; p = 0.274).
Table 1. Patient and donor characteristics1
|
ALL, acute lymphoblastic leukemia; B-ALL, B-cell ALL; CR1, first complete remission; CR2, second complete remission; HIDT, haploidentical donor transplantation; HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplantation; MSDT; HLA-matched sibling donor transplantation; Ph, Philadelphia-chromosome; MRD, measurable residual disease; T-ALL, T-cell ALL. |
|||
|
Characteristics |
HIDT group |
MSDT group |
p value |
|---|---|---|---|
|
Number of patients |
169 |
39 |
|
|
Median age, years (range) |
24 (3–58) |
35 (9–60) |
0.001 |
|
Male sex, n (%) |
107 (63.3) |
21 (53.8) |
0.273 |
|
Diagnosis, n (%) |
|
|
|
|
B-ALL: |
— |
— |
0.142 |
|
Ph-positive |
49 (29.0%) |
10 (25.6%) |
|
|
Ph-negative |
120 (55.0%) |
29 (69.2%) |
|
|
T-ALL |
27 (16.0%) |
2 (5.1%) |
|
|
Disease status, n (%) |
|
|
0.328 |
|
CR1 |
131 (77.5) |
33 (84.6) |
|
|
≥ CR2 |
8 (22.5) |
6 (15.4) |
|
|
ABO matched grafts, n (%) |
|
|
0.414 |
|
Matched |
98 (58.0) |
19 (48.7) |
|
|
Major mismatch |
30 (17.8) |
8 (20.5) |
|
|
Minor mismatch |
33 (19.5) |
11 (28.2) |
|
|
Bi-directional mismatch |
8 (4.7) |
1 (2.6) |
|
|
Intervention for positive MRD post-HSCT among all patients, n (%) |
39 (21) |
16 (41) |
0.027 |
|
Intervention among positive MRD post-HSCT patients, n (%) |
39/45 (87) |
16/17(94) |
0.662 |
Table 2. Transplant outcomes between patients who underwent HIDT and those who received MSDT1
|
CI, confidence interval; GRFS, GvHD-free, relapse-free survival; GvHD, graft-versus-host disease; HIDT, haploidentical donor transplantation; HLA, human leukocyte antigen; LFS, leukemia-free survival; MRD, measurable residual disease; MSDT, HLA-matched sibling donor transplantation; NRM, non-relapse mortality; OS, overall survival. |
|||
|
Parameter |
HIDT group, % (95% CI) |
MSDT group, % (95% CI) |
p value |
|---|---|---|---|
|
Grades II–IV acute GvHD |
21 (17–27) |
23 (10–36) |
0.884 |
|
Total chronic GvHD |
44 (36–52) |
48 (31–65) |
0.850 |
|
Moderate-to-severe chronic GvHD |
18 (10–26) |
27 (10–44) |
0.192 |
|
Cumulative incidence of positive MRD after transplantation |
26 (19–33) |
44 (28–60) |
0.043 |
|
3-year probability of relapse |
23 (17–29) |
47 (31–63) |
0.006 |
|
3-year probability of NRM |
11 (6–16) |
10 (1–19) |
0.845 |
|
3-year probability of LFS |
65 (58–72) |
43 (27–59) |
0.023 |
|
3-year probability of OS |
68 (61–75) |
46 (30–62) |
0.039 |
|
3-year probability of GRFS |
54 (46–62) |
36 (21–51) |
0.055 |
Table 3. Uni- and multivariate analysis of factors associated with transplantation outcomes (n = 208)1
|
CI, confidence interval; CR1, first complete remission; CR2, second complete remission; GvHD, graft-versus-host disease; HIDT, haploidentical donor transplantation; HLA, human leukocyte antigen; HR, hazard ratio; MSDT, HLA-matched sibling donor transplantation; MRD, measurable residual disease. * All variables were first included in the univariate analysis; only variables with P<0.1 and the forced variable (transplant modality) were included in the Cox proportional hazards model with time-dependent variables. Bold font indicates statistical significance. |
||||||
|
Covariate* |
Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
|
|
HR |
95% CI |
p value |
HR |
95% CI |
p value |
|
Relapse |
|
|
|
|
|
|
|
Disease status (≥ CR2 vs CR1) |
2.356 |
1.343–4.134 |
0.003 |
2.528 |
1.357–4.707 |
0.003 |
|
Levels of pre-transplantation MRD |
2.204 |
1.267–3.835 |
0.005 |
1.320 |
1.060–1.642 |
0.013 |
|
Chronic GvHD (yes vs no) |
0.475 |
0.263–0.859 |
0.014 |
0.337 |
0.181–0.628 |
0.001 |
|
Post-transplantation MRD |
2.168 |
1.283–3.664 |
0.004 |
2.149 |
1.253–3.685 |
0.005 |
|
Transplant modality (HIDT vs MSDT) |
0.465 |
0.265–0.815 |
0.008 |
0.360 |
0.197–0.655 |
0.001 |
|
Non-relapse mortality |
|
|
|
|
|
|
|
Platelet engraftment (yes vs no) |
0.038 |
0.015–0.097 |
< 0.001 |
0.048 |
0.018–0.122 |
< 0.001 |
|
Grades II–IV acute GvHD |
3.382 |
1.481–7.723 |
0.004 |
2.573 |
1.102–6.008 |
0.029 |
|
Transplant modality (HIDT vs MSDT) |
1.095 |
0.372–3.218 |
0.869 |
0.663 |
0.213–2.061 |
0.478 |
|
Overall survival |
|
|
|
|
|
|
|
Disease status (≥ CR2 vs CR1) |
2.311 |
1.404–3.806 |
0.001 |
2.238 |
1.321–3.790 |
0.003 |
|
Levels of pre-transplantation MRD |
1.312 |
1.107–1.555 |
0.002 |
1.202 |
0.975–1.482 |
0.085 |
|
Platelet engraftment (yes vs no) |
0.077 |
0.036–0.169 |
< 0.001 |
0.083 |
0.034–0.199 |
< 0.001 |
|
Grades II–IV acute GvHD |
2.555 |
1.559–4.185 |
< 0.001 |
2.426 |
1.442–4.082 |
0.001 |
|
Chronic GvHD (yes vs no) |
0.482 |
0.292–0.794 |
0.004 |
0.469 |
0.269–0.820 |
0.008 |
|
Post-transplantation MRD |
1.438 |
0.915–2.259 |
0.116 |
1.649 |
0.990–2.746 |
0.055 |
|
Transplant modality (HIDT vs MSDT) |
0.584 |
0.348–0.980 |
0.042 |
0.395 |
0.225–0.695 |
0.001 |
Table 4. Primary cause of death among patients that underwent allogeneic stem cell transplantation1
|
GvHD, graft-versus-host disease; HIDT, haploidentical donor transplantation; HLA, human leukocyte antigen; MSDT, HLA-matched sibling donor transplantation. |
||
|
Cause of death |
HIDT group, n (%) |
MSDT group, n (%) |
|---|---|---|
|
Relapse |
32 (62.7) |
16 (80.0) |
|
Infection |
12 (23.5) |
2 (10.0) |
|
Graft failure |
3 (5.9) |
0 (0) |
|
GvHD |
1 (2.0) |
2 (10.0) |
|
Others |
3 (5.9) |
0 (0) |
Conclusion
Based on the outcome of this prospective phase III study, HIDT is preferred over MSDT (regardless of available matched sibling donors) for patients with pretransplant MRD-positive ALL, in view of favorable anti-leukemia activity of cells derived from haploidentical donors. Furthermore, disease status (≥ CR2), development of chronic GvHD, and use of MSDT predicted inferior leukemia-free survival, cumulative incidence of relapse, and OS in a multivariate analysis. These findings could inform decision making and the development of donor-selection algorithms.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

