All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Ponatinib versus imatinib in combination with low-intensity chemotherapy in patients with newly-diagnosed Ph+ ALL: PhALLCON trial
BCR-ABL1 tyrosine kinase inhibitors (TKI) plus chemotherapy or steroids are the current standard-of-care first line treatment for Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL).1 Ponatinib is the only pan-inhibitory BCR-ABL1 TKI with activity against BCR-ABL1 wild-type and single mutation variants, and therefore, may improve long-term outcomes compared with other BCR-ABL1 TKIs. PhALLCON (NCT03589326) is an international trial comparing the efficacy and safety of ponatinib vs imatinib combined with low-intensity chemotherapy in adult patients with de novo Ph+ ALL.1
Study design
This phase III, multicenter trial enrolled patients with de novo Ph+ ALL or BCR-ABL1 positive ALL.1 Eligibility patients were:
- ≥18 years of age;
- Had an Eastern Cooperative Oncology Group performance status of 0 to 2;
- Absence of history or current diagnosis of chronic phase-chronic myeloid leukemia (CML), accelerated phase CML, or B-lymphoid blast phase CML; and
- Absence of clinically significant or uncontrolled cardiovascular disease.
Patients were randomized to either ponatinib + reduced intensity chemotherapy or imatinib + reduced intensity chemotherapy.1 The treatment schema is shown in Figure 1.
The primary endpoint was measurable residual disease (MRD)-negative complete remission (CR) (defined as hematologic CR ≥4 weeks) in combination with MRD negativity (≤0.01% BCR-ABL1/ABL1 transcripts).1 Key secondary endpoints were event-free survival, progression-free survival, and safety.
Figure 1. Treatment schema*
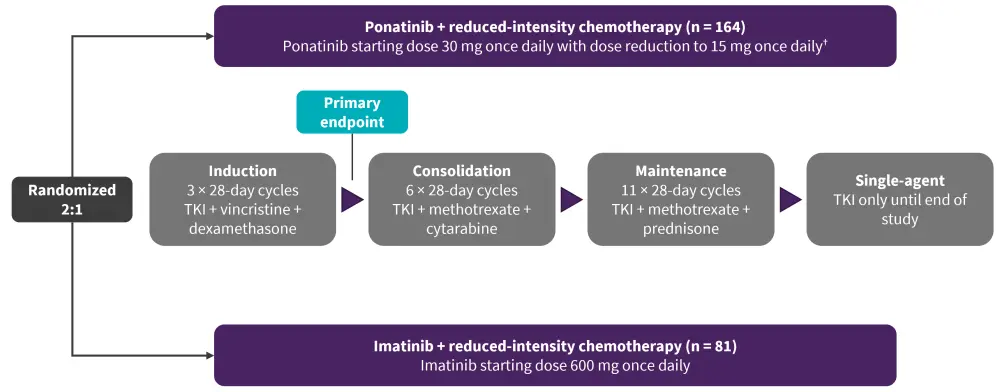
CR, complete response; MRD, minimal residual disease; TKI, tyrosine kinase inhibitor.
*Adapted from Jabbour.1
†Dose reduction to 15 mg once daily were implemented in patients who achieved MRD-negative CR after completion of the induction phase.
Results
At a median follow-up of 20.4 vs 18.1 months in the ponatinib and imatinib arm, more patients in the ponatinib arm continued to receive treatment at 41% and 12%, respectively.1 The baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics*
|
CV, cardiovascular; ECOG, Eastern Cooperative Oncology Group. |
||
|
Characteristic, % (unless otherwise stated) |
Ponatinib |
Imatinib
|
|---|---|---|
|
Median age, years (range) |
54 (19–82) |
52 (19–75) |
|
≥60 years |
37 |
37 |
|
Male |
45 |
47 |
|
ECOG Performance Status of 0 or 1 |
96 |
94 |
|
Median leukocyte count (range), ×109/L |
4.4 (0.4–198) |
3.2 (0.2–81) |
|
Median leukocyte blasts (range) |
80 (0–100) |
75 (0–100) |
|
Cardiovascular morbidity |
|
|
|
≥1 CV morbidity |
56 |
64 |
|
≥2 CV morbidity |
28 |
33 |
|
BCR-ABL1 dominant variant |
|
|
|
p190 |
70 |
65 |
|
p210 |
24 |
31 |
Efficacy
Efficacy data was available for 154 and 78 patients in the ponatinib and imatinib arm, respectively.1 The primary endpoint of MRD-negative CR at the end of induction was met, with ponatinib showing a two-fold increase in MRD-negative CR compared with imatinib (Figure 2). After each cycle of BCR::ABL1 ≤0.01%, the MRD-negativity response rate increased continuously and was higher in patients treated with ponatinib compared with imatinib (Figure 3). Patients receiving subsequent second- or third-generation TKI and/or immunotherapy were higher in the imatinib versus ponatinib arm (37% vs 19%). Median event-free survival was not met in the ponatinib arm compared to 29 months in the imatinib arm (hazard ratio, 0.65; 95% confidence interval [CI], 0.39–1.10) and median progression-free survival was 20 months in the ponatinib-treated arm (95% CI, 11.8–NE) compared with 7.9 months in the imatinib-treated arm (95% CI, 6.2–12).1
Figure 2. Primary endpoint efficacy outcomes*
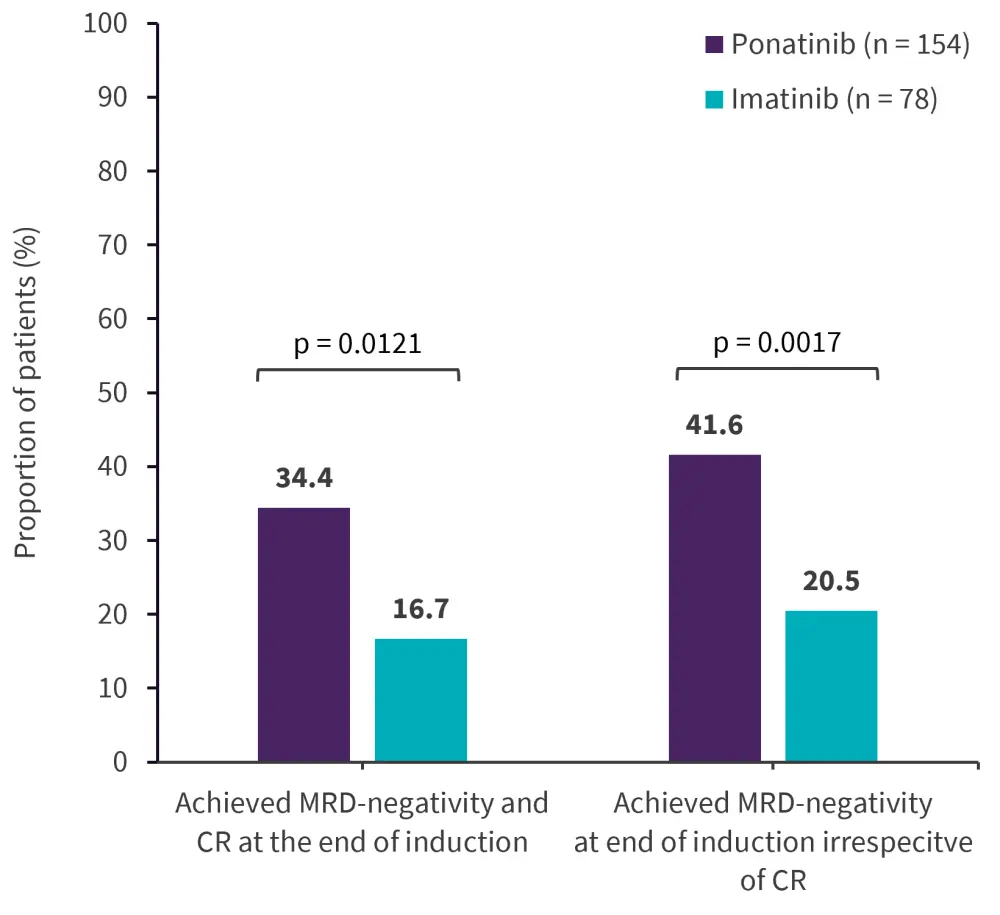
CR, complete remission; MRD, measurable residual disease.
*Adapted from Jabbour.1
Figure 3. Efficacy outcomes BCR:ABL1 ≤0.01% during Cycles 3–9*
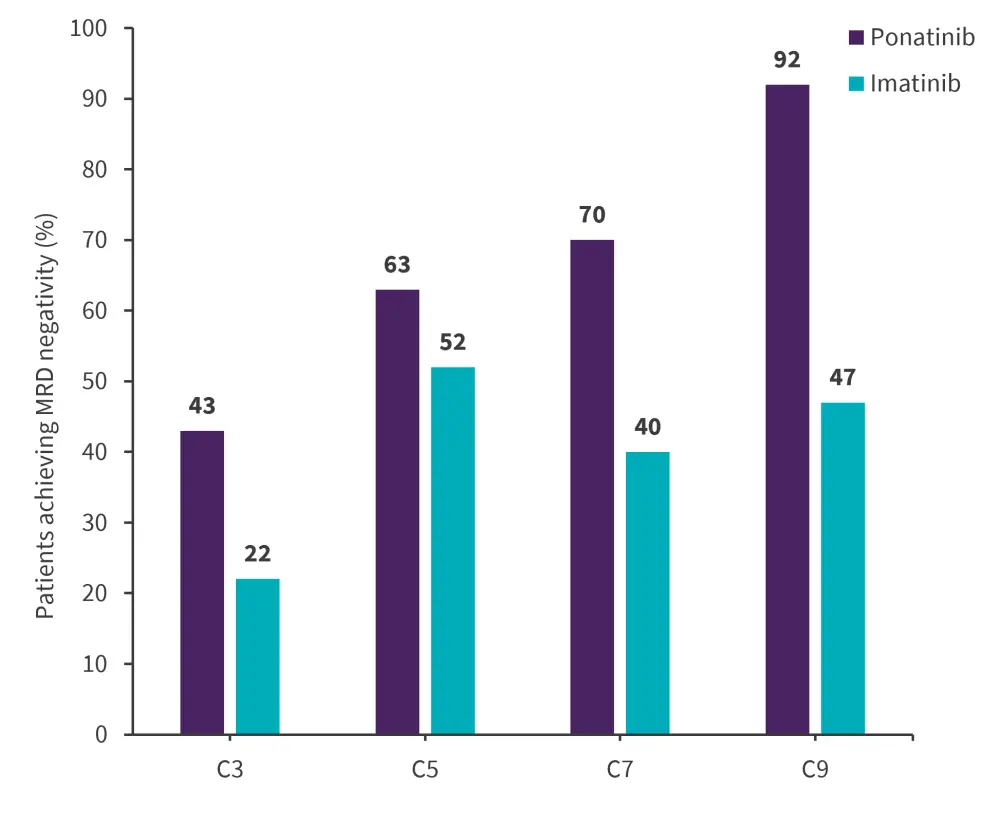
C, cycle.
*Adapted from Jabbour.1
Safety
Safety data was available for 163 and 81 patients in the ponatinib and imatinib arm, respectively.1 A higher number of patients in the imatinib arm discontinued treatment compared with the ponatinib arm (86% vs 58%). Serious treatment-emergent adverse events (TEAE) were reported in 60% vs 56% of patients in the ponatinib and imatinib arms, respectively.1
- In the ponatinib arm, Grade 3-4 and Grade 5 serious TEAEs were reported in 90% and 5% of patients, respectively.
- In the imatinib arm, Grade 3-4 TEAEs were reported in 93% of patients and Grade 5 were reported in 5% of patients.
- Grade 5 TEAEs reported in the ponatinib arm were septic shock (n = 4), abdominal sepsis, sepsis, pneumonitis, and respiratory failure (n = 1 each).
- Grade 5 TEAEs reported in the imatinib arm were septic shock, pseudomembranous colitis, pulmonary sepsis, and depressed level of consciousness (n = 1 each).
Conclusion
The PhALLCON trial reached its primary endpoint and demonstrated a higher MRC-negative CR rate at the end of induction compared with imatinib.1 In addition, the safety profile of ponatinib was comparable to imatinib. These findings suggest that ponatinib combined with low-intensity chemotherapy appears to be a more effective therapy in patients with newly diagnosed Ph+ ALL and has the potential to be standard of care in this population.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


