All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Prognostic impact of NGS MRD and its correlation with RT-PCR for BCR::ABL1 in Ph-positive ALL
Do you know... Among the 29 patients with Philadelphia-chromosome positive acute lymphoblastic leukemia in the early MRD group of the retrospective group, what was the concordance rate between NGS MRD and RT-PCR for BCR::ABL1?
Minimal residual disease (MRD) is a prognostic biomarker for relapse and long-term survival outcomes in acute lymphoblastic leukemia (ALL). Achievement of a complete molecular response (CMR) is associated with better outcomes in patients with Philadelphia-chromosome positive (Ph+) ALL receiving frontline therapy, and failure to achieve a CMR is associated with high relapse rates.1
Although reverse transcription polymerase chain reaction (RT-PCR) is recommended for MRD assessments in Ph+ ALL, it is not the optimal method for predicting the risk of relapse, with around 20–30% of patients still experiencing relapse after achieving a CMR and some achieving long-term survival despite persistent MRD. Additionally, even with a similar sensitivity assay, it is poorly correlated with other methods such as flow cytometry, RT-PCR for immunoglobulin (IG), or T-cell receptor (TR) rearrangements.1
Next-generation sequencing (NGS) is an MRD assessment method which has a higher sensitivity to RT-PCR assays and a limit of detection of 10−6. Whilst the prognostic use of this method has been established in Philadelphia chromosome-negative ALL, its clinical impact has not been systematically investigated in patients with Ph+ ALL. The ALL Hub previously reported on the prognostic significance of MRD status in patients with ALL undergoing allogeneic hematopoietic stem cell transplant later than first complete remission. Here, we summarize an article by Short et al.1 published in American Journal of Hematology on the prognostic impact of NGS MRD for IG/TR and its correlation with RT-PCR for BCR::ABL1 in Ph+ ALL.
Study methods
This retrospective study included patients with Ph+ ALL who received frontline therapy with a hyper-CVAD (hyperfractionated-cyclophosphamide, vincristine, doxorubicin, and dexamethasone) based regimen, plus a BCR::ABL1 tyrosine kinase inhibitor at the MD Anderson Cancer Center. Pretreatment bone marrow samples included for analysis were:
- Early MRD group: remission marrow collected between 1 and 6 months from the start of frontline therapy
- Long-term PCR-negative group: remission marrow collected >2 years from the start of treatment for patients who were in CMR for at least 1 year
- Long-term PCR-positive group: remission marrow collected >2 years from the start of treatment for those with persistent MRD by PCR for at least 1 year
The sensitivity of the BCR::ABL1 RT-PCR assessment was between 10−4 and 10−5 and the clonoSEQ NGS-based MRD assessment used a sensitivity level of 10−6.
Endpoints
- CMR, defined as the absence of a quantifiable BCR::ABL1 transcript by RT-PCR
- Relapse, defined as the recurrence of bone marrow blasts at >5% or extramedullary ALL
- Relapse-free survival (RFS), defined as the time from response until relapses or death from any cause
- Overall survival (OS), defined as the time from the start of treatment until death from any cause
Results
Baseline characteristics
Overall, 44 patients were included in the study, 30 of whom had the p190 BCR::ABL1 isoform and 14 with the p210 isoform. The median number of dominant sequences per baseline sample was two (range, 1−6 sequences) and the median follow-up was 84 months. Baseline characteristics are summarized in Table 1.
Table 1. Baseline characteristics*
|
CNS, central nervous system; HSCT, hematopoietic stem cell transplantation; PCR, polymerase chain reaction; WBC, white blood cell. |
|||
|
Characteristic, % (unless otherwise stated) |
Early PCR group† |
Long-term PCR− group |
Long-term PCR+ group |
|---|---|---|---|
|
Median age (range), years |
46 (24–77) |
51 (31–80) |
57 (28–73) |
|
WBC, ×109/L |
21.8 (0.4–629.4) |
15.1 (0.4–229.1) |
23.2 (3.0–105.6) |
|
Bone marrow blasts |
77 (8–93) |
81 (32–93) |
84 (61–96) |
|
CNS involvement at diagnosis |
14 |
10 |
0 |
|
Transcript type |
|
|
|
|
p190 |
66 |
70 |
75 |
|
p210 |
34 |
30 |
25 |
|
Tyrosine kinase inhibitor |
|
|
|
|
Imatinib |
0 |
5 |
0 |
|
Dasatinib |
48 |
60 |
25 |
|
Ponatinib |
52 |
35 |
75 |
|
HSCT in first remission |
21 |
0 |
0 |
Retrospective cohort results
Early MRD group: correlation between BCR::ABL1 RT-PCR and NGS MRD
Among the 29 patients who had an NGS MRD assessment in the early MRD group, 46 samples were evaluated for NGS MRD.
- The concordance between NGS and RT-PCR based testing was 68% and the discordance rate was 32%.
- For 16 out of the 17 samples that were NGS+/PCR+, the median level of RT-PCR for BCR::ABL1 was significantly higher than that of NGS MRD (p = 0.001).
- Of the 25 patients with a NGS MRD assessment at 10-6, eight were discordant, five with PCR+/NGS- and three with PCR-/NGS+. The concordance and discordance rates of RT-PCR for BCR::ABL1 and NGS for IG/TR are reported in Figure 1.
- Patients who achieved NGS MRD negativity in the first 6 months of treatment had a significantly higher 5-year RFS and OS rates than those who achieved NGS MRD positivity (Figure 2).
Figure 1. Concordance and discordance rates in the early MRD group*
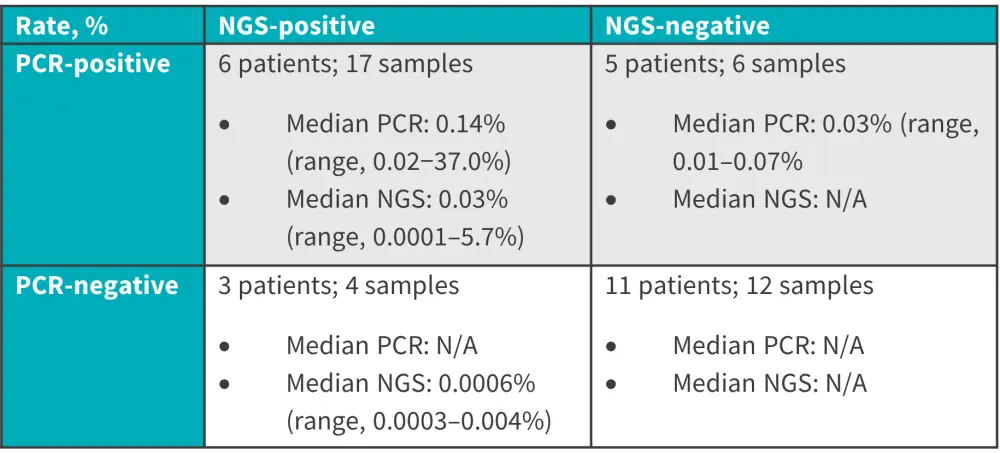
MRD, minimal residual disease; N/A, not available; NGS, next-generation sequencing; PCR, polymerase chain reaction.
*Data from Short, et al.1
Figure 2. 5-year responses in the early MRD group*
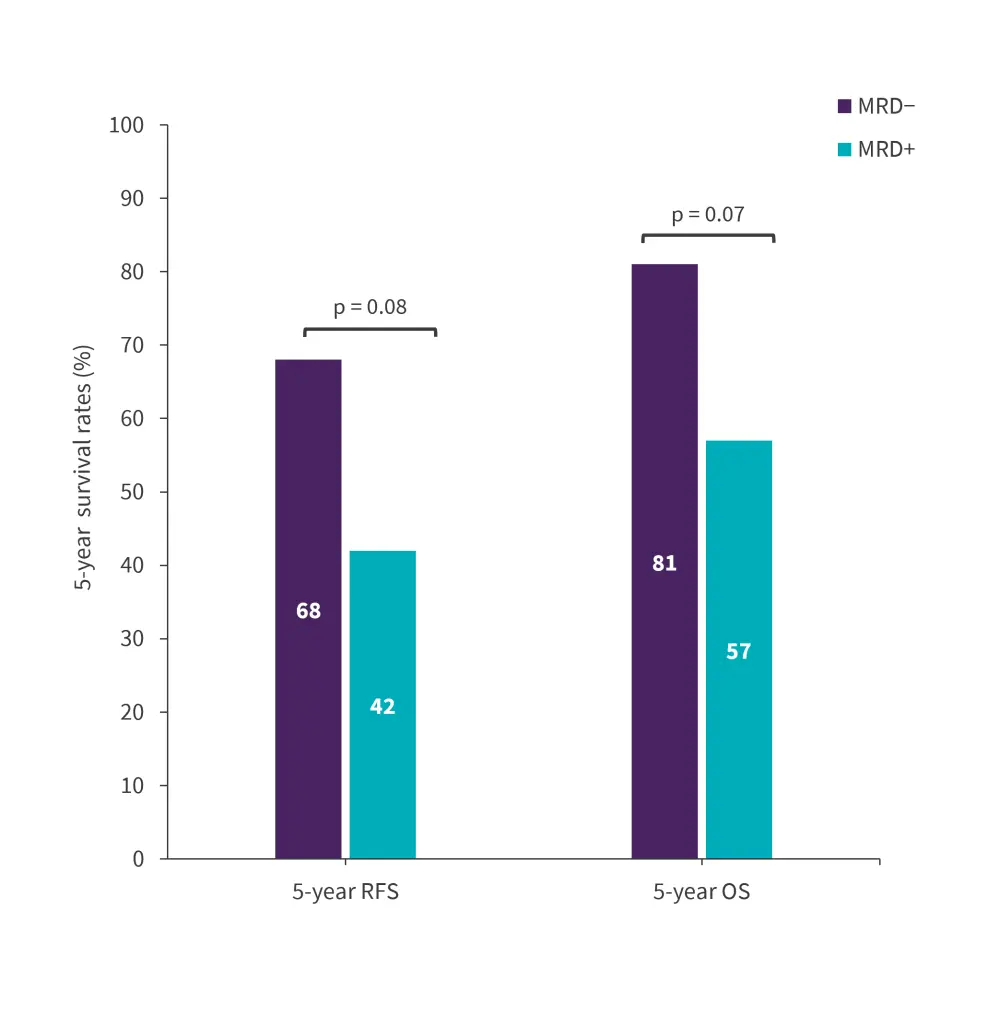
MRD, minimal residual disease; OS, overall survival; RFS, relapse-free survival.
*Data from Short, et al.1
Long-term PCR group: Correlation and impact of NGS MRD
Among the 20 patients in the long-term PCR-negative group, 20 remission samples were evaluated for NGS MRD. The median follow-up after assessments was 58 months. A total of 18 patients were NGS MRD negative at a sensitivity level of 10-6, and none were NGS MRD positive. One out of the 20 patients who received frontline hyper-CVAD plus ponatinib and achieved long-term CMR experienced disease relapse.
Among the eight patients in the long-term PCR-positive group, 21 samples were evaluated for NGS MRD, with a median time from the start of therapy to NGS MRD assessment of 34 months (range, 25–85 months).
- Six patients were NGS MRD negative and two patients were NGS MRD positive at a level of 10−6
- Among the 13 PCR+/NGS- samples in six patients, median PCR value was 0.03% (range, 0.01–17.68%)
- At a median follow-up of 70 months, none of the six patients with PCR+/MRD- relapsed
- Due to persistent MRD, two patients with PCR+/MRD- underwent treatment intervention; one received ponatinib plus blinatumomab followed by investigational CD19 chimeric antigen receptor T-cells and remains in ongoing remission for >60 months and the other patient received ponatinib plus blinatumomab and has remained in continuous remission for >50 months.
- Among the two patients with PCR+/NGS+, the median NGS MRD level was 0.0001% and the median PCR MRD value was 0.04% (range, 0.01–0.07%).
- Both patients with PCR+/NGS+ received treatment interventions; one was treated with ponatinib plus blinatumomab, achieved CMR, and has remained in continuous CMR for >5 years. The second patient received ponatinib plus blinatumomab achieved CMR but experienced disease relapse 8 months later.
Prospective validation cohort results
Of the 74 patients with Ph+ ALL in the prospective validation, 65 achieved NGS MRD negativity at a level of 10−6. Overall, 11 out of the 65 patients were also MRD positive by PCR; baseline characteristics for these patients are summarized in Table 2.
Table 2. Baseline characteristics of the prospective validation cohort*
|
CR, complete remission; HSCT, hematopoietic stem cell transplantation; Hyper-CVAD, hyperfractionated-cyclophosphamide, vincristine, doxorubicin, and dexamethasone. |
|
|
Characteristic, % (unless otherwise stated) |
Patients |
|---|---|
|
Median age (range), years |
51 (23–79) |
|
Transcript type |
|
|
p190 |
91 |
|
p210 |
9 |
|
Tyrosine kinase inhibitor |
|
|
Hyper-CVAD plus imatinib |
9 |
|
Hyper-CVAD plus dasatinib |
9 |
|
Hyper-CVAD plus ponatinib |
18 |
|
Blinatumomab plus dasatinib |
9 |
|
Blinatumomab plus ponatinib |
55 |
|
HSCT in CR1 |
0 |
The median PCR value for patients with MRD PCR+/NGS- in the validation cohort was 0.05% (range, 0.01%–1.23%). Due to persistent PCR MRD, two patients underwent treatment interventions; one received ponatinib plus inotuzumab ozogamicin and remains PCR+/NGS- on ponatinib monotherapy, and the other patient received ponatinib, venetoclax, plus blinatumomab and remains PCR+/NGS on ponatinib plus venetoclax.
At a median follow-up of 11 months, none of the 11 patients relapsed. The 5-year RFS and OS rates were similar for the 20 patients who were PCR+/NGS− and the 22 patients who were PCR−/NGS− in the retrospective primary and validation cohort combined (Figure 3).
Figure 3. 5-year responses for the combined cohorts*
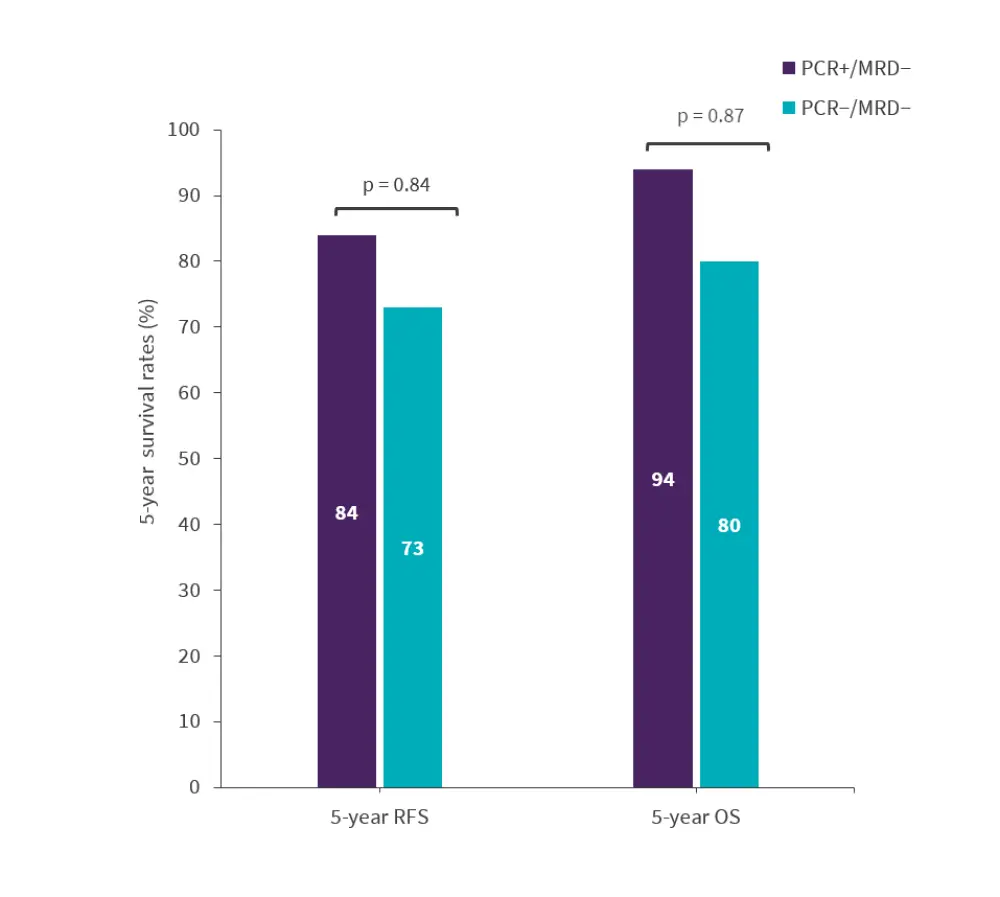
MRD, minimal residual disease; OS, overall survival; PCR, polymerase chain reaction; RFS, relapse-free survival.
*Data from Short, et al.1
Conclusion
Consistent with studies on NGS MRD in Ph-negative ALL, NGS MRD was prognostic for RFS and OS in patients with Ph+ ALL. Overall, these data suggest that RT-PCR for BCR::ABL1 is not prognostic in patients who achieve NGS MRD negativity, but NGS MRD negativity can identify patients at very low risk of relapse and who did not appear to benefit from therapeutic interventions despite a persistent MRD detected by RT-PCR, and NGS MRD may be able to better guide treatment decision than the conventional used RT-PCR for BCR::ABL1. However, the question of whether NGS MRD could replace RT-PCR in Ph+ ALL will be determined in future prospective studies.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


