All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Progress and challenges of CAR T-cell therapies for T-cell leukemia and lymphoma
Question 1 / 1
Which of the following methods are NOT used to mitigate T-cell aplasia during the use of CAR T-cell therapies for T-cell malignancies?
A
Drug-inducible switches
B
Dual-targeted CAR T-cell therapies
C
Naturally selected CAR T-cells
D
Using CAR T-cell therapy as a bridge to allogeneic stem cell transplantation
Although chimeric antigen receptor (CAR) T-cell therapies have revolutionized the treatment landscape for relapsed/refractory (R/R) B-cell malignancies, their use in both children and adults with T-cell leukemias and lymphomas remains limited. This aggressive disease is often refractory to standard chemotherapy, and there can be challenges related to CAR T-cell therapy design, manufacturing, expansion, and tumor biology.1
The ALL Hub has previously reported on the progress of targeted therapies and immunotherapies for T-cell acute lymphoblastic leukemia/lymphoma (T-ALL/LBL), with a recent expert opinion on whether CAR T-cell therapy is a safe and effective treatment option for R/R T-ALL and lymphoma.
Here, we summarize the review published by Angelos et al.1 in Transplantation and Cellular Therapy on the progress and challenges of CAR T-cell therapies for patients with T-cell leukemia/lymphoma, including novel strategies to overcome these barriers and the preliminary efficacy of different CAR T-cell products for this patient population.
Clinical efficacy of CAR T-cells in T-cell malignancies
Several CAR T-cell strategies have achieved promising results in patients with T-cell malignancies, including autologous anti-CD5 CAR T-cells for patients with R/R T-cell non-Hodgkin lymphoma or anti-CD5 CAR T-cell manufactured with tyrosine kinase inhibitors for patients with R/R T-ALL (MAGENTA trial, NCT03081910), donor-derived anti-CD7 CAR T-cell therapy for pediatric and adult patients with R/R T-ALL (ChiCTR2000034762), autologous naturally selected anti-CD7 CAR T-cells in patients with R/R T-ALL/LBL (NCT04572308), and anti-CD30 CAR T-cells with CRISPR/Cas9 deletion of TRAC and CD70 for patients with R/R T-cell lymphoma (COBALT-LYM trial, NCT04502446) (Figure 1).
Figure 1. Preliminary results from ongoing clinical trials investigating CAR T-cell therapies in T-cell malignancies*
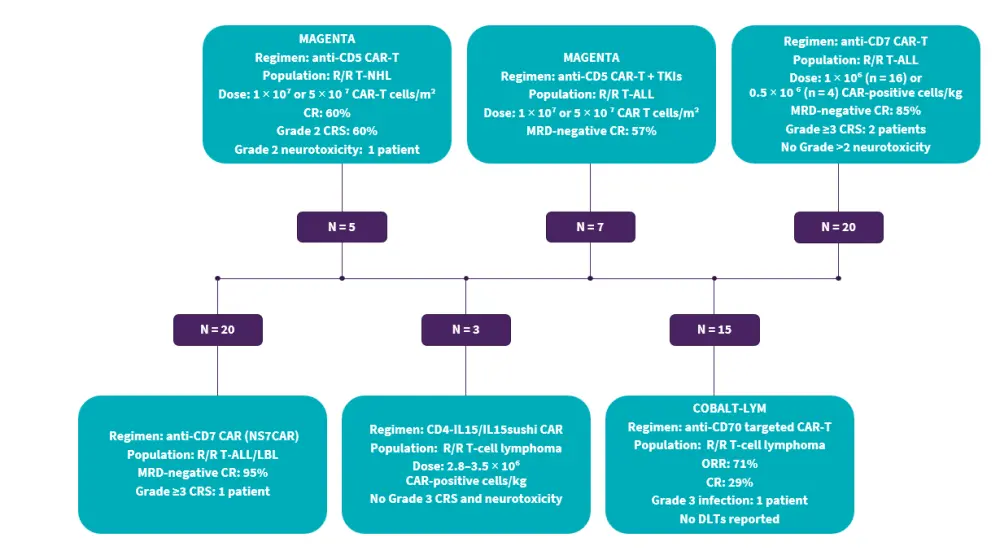
ALL, acute lymphoblastic leukemia; CAR, chimeric antigen receptor; CR, complete remission; CRS, cytokine release syndrome; DLT, dose-limiting toxicity; MRD, measurable residual disease; NHL, non-Hodgkin lymphoma; ORR, overall response rate; R/R, relapsed/refractory; T-ALL/LBL, T-cell acute lymphoblastic leukemia/lymphoblastic leukemia; TKI, tyrosine kinase inhibitor.
Data from Angelos, et al.1
Other CAR T-cell products currently undergoing early-phase clinical investigation for T-cell malignancies include: CART5, an autologous CD5 CRISPR-Cas9 knockout anti-CD5 CAR T-cell product in phase I development for patients with R/R CD5-expressing nodal T-cell lymphomas; UCART7, an allogeneic, fratricide-resistant, anti-CD7 CAR T-cell product incorporating dual TRAC and CD7 multiplexed gene deletion in a phase 1/2 study for patients with R/R T-ALL/LBL (NCT04984356); and anti-CD4 directed CAR T-cells in a phase I multicenter study for patients with R/R T-cell leukemia and lymphoma (NCT03829540).
Challenges of CAR T-cell therapies in T-cell malignancies
In addition to the barriers observed with CAR T-cells for B-cell malignancies, new challenges seen in CAR T-cells for T-cell malignancies include fratricide, production contamination, T-cell aplasia, and clonal escape (Figure 2).
Figure 2. Challenges in the use of CAR T-cell therapy for T-cell malignancies*
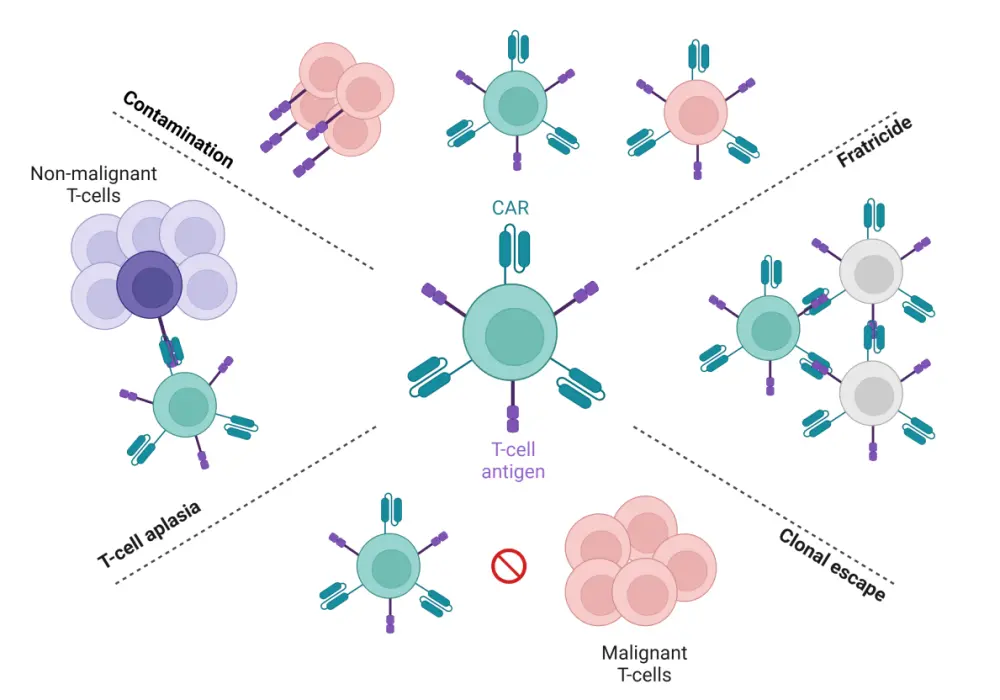
CAR, chimeric antigen receptor.
Data from Angelos, et al.1
Created with BioRender.com.
Fratricide
Fratricide is a phenomenon when CAR T-cells not only target malignant T-cells but also target other CAR T-cells that express the target antigen. Fratricide prevents adequate CAR T-cell expansion necessary for a clinical benefit. Given the lack of T-cell tumor-specific antigens, most CAR T-cell products to date use pan-T-cell antigens highly expressed on malignant T-cells such as CD3, CD5, and CD7. While the use of CD7- and CD3-targeted CAR T-cells results in complete fratricide, CD5-directed CAR T-cells can partially overcome the issue of fratricide, possibly owing to rapid internalization upon ligand binding in effector T-cells and epitope masking of the CD5 extracellular domain.
Product contamination
The risk of CAR T-cell product contamination with malignant T-cells is substantially higher in T-cell malignancies compared with B-cell malignancies; this is compounded by the purification of malignant and non-malignant T-cells without a distinguishable surface antigen in the autologous setting, a lack of understanding of the lowest limit of measurable residual disease (MRD) needed that can limit product contamination, as well as low sensitivity of methods to detect MRD when segregating non-malignant vs malignant cells. As such, autologous CAR T-cell products are not recommended for patients with positive MRD and circulating T-cell malignancies.
T-cell aplasia
CAR T-cell therapies have the potential to induce on-target off-tumor effects against non-malignant T-cells, increasing the risk of severe infections during the treatment of T-cell malignancies. While prolonged T-cell aplasia or Grade 3 or 4 infection-related adverse events have not been reported in clinical cases of T-cell directed CAR T-cell therapy, the potential life-threatening infectious risks prompt the use of prolonged anti-bacterial, viral, fungal agents, as well as preemptive monitoring for cytomegalovirus and Epstein-Barr virus in pan-T-cell antigen-specific CAR T-cell regimens.
Clonal escape
Clonal escape can cause CAR T-cell therapy failure at any point after CAR T-cell infusion through several mechanisms, including antigen escape, antigen downregulation, and lineage switching. Antigen escape commonly occurs due to target-antigen-negative malignant subclone expansion or, in some cases, due to a point mutation within the target antigen. Antigen downregulation arises when there is a reduction in the total target-antigen density on tumor surface or through epitope masking.
Proposed solutions to overcome challenges of CAR T-cells in T-ALL/LBL
To overcome fratricide, strategies include deleting the target antigens on CAR T-cells using genome editing; however, one of the problems that remain is the inability to delete important targets for T-cell growth, survival, and optimal cytotoxic CAR T-cell function such as CD3, CD8, or CD30. CRISPR-Cas9 base editing can be used to delete unessential T-cell antigens alongside standard CAR transduction methods to develop fratricide-resistant CAR T-cell products. Moreover, drug-induced genetic switches can also be employed to induce temporospatial CAR expression on the extracellular membrane and prevent fratricide.
One approach to eradicate product contamination and the risk of malignant cell transduction is the use of allogeneic CAR T-cells from healthy donors, which can allow enrichment with highly active cytotoxic CAR T-cells while avoiding manufacturing issues; however, given the T-cell receptor and major histocompatibility complex incompatibility, this procedure may cause graft-versus-host disease. Deletion of either the TRAC or TRBC genes can prevent alloreactivity without compromising the CAR T-cell response.
Non-T CAR cell or CARs derived from less common immune cells such as gamma-delta T-cells, natural killer (NK) cells, invariant NK cells, and macrophages are other strategies in early-phase clinical trials that can prevent product contamination. There are limited clinical data on the use of CAR-invariant NK cells and CAR-gamma delta T-cells, but CAR NK-cells have demonstrated preclinical efficacy in both in vitro and in vivo cancer models.
Strategies to limit T-cell aplasia are similar to those used to mitigate fratricide, including the incorporation of drug-inducible switches or genome editing. Another feasible solution to address T-cell aplasia is using CAR T-cell therapy as a bridge to allogeneic hematopoietic stem cell transplantation to allow for complete adaptive immune cell reconstitution following tumor debulking. Further to this, naturally selected CAR T-cells through clonal expansion of T-cell antigen-deficient subpopulations or direct manufacturing of CAR-negative T-cells can also be used to prevent fratricide and T-cell aplasia (Figure 3).
To decrease the probability of antigen escape, dual- and tandem-expressing CARs can be used to target multiple T-cell antigens; tandem anti-CD5 and anti-CD7 and dual-targeted anti CD3/CD7 have demonstrated preclinical efficacy. Key points to consider during this process include the deletion efficiency of multiplexed T-cell antigen genes and the size of CAR constructs that maintain an adequate T-cell transduction efficiency.
Figure 3. Overview of strategies to overcome the challenges of using CAR T-cell therapy*
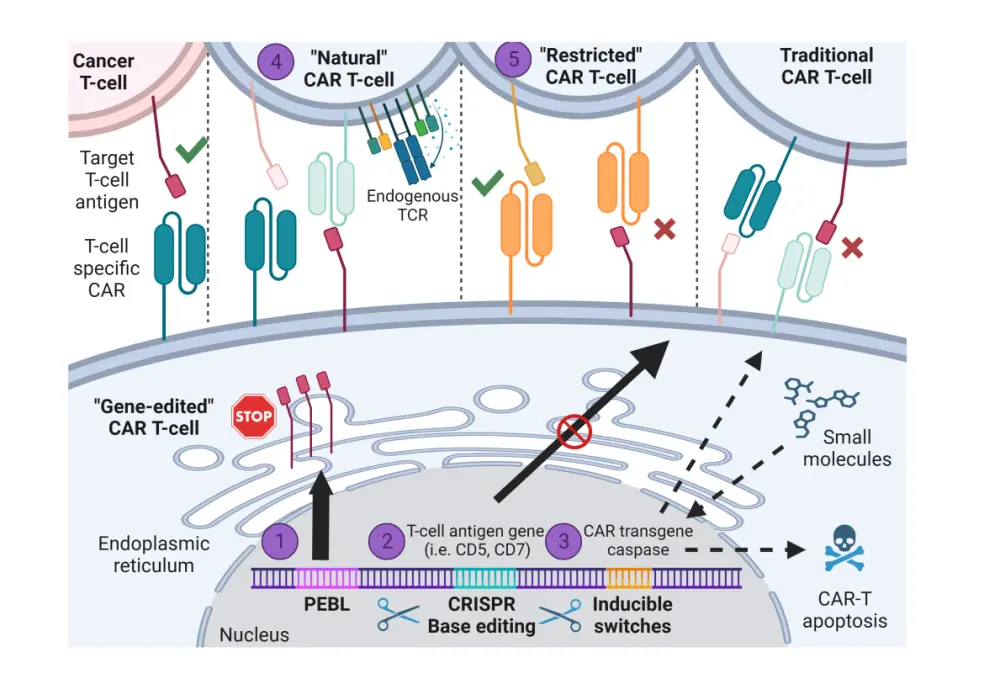
CAR, chimeric antigen receptor; CRISPR, clustered regularly interspaced short palindromic repeats; PEBL, protein expression blocker; TCR, T-cell receptor.
Data from Angelos, et al.1
Created with BioRender.com.
Conclusion
This review highlights the clinical challenges of fratricide, T-cell aplasia, product contamination, and clonal escape associated with CAR T-cell treatment for T-cell leukemias and lymphomas, as well as novel strategies to circumvent these barriers. Approaches such as drug-inducible switches, base editing, and naturally selected CAR T-cells can be used to prevent both fratricide and T-cell aplasia; allogeneic CAR T-cells can prevent product contamination, and the use of dual-targeted or tandem CAR T-cells can reduce likelihood of clonal escape.
Anti-CD5-, CD4-, CD70-, and CD7-directed CAR T-cells have demonstrated preliminary efficacy for high-risk patients with T-cell leukemias and lymphomas and will likely further improve outcomes in the near future.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

