All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Risk factors for induction-related mortality in AYA and adult patients with ALL in resource-constrained settings
Acute lymphoblastic leukemia (ALL) is characterized by malignant clonal expansion of lymphoid progenitors in the bone marrow and high mortality rates during initial treatment. Diagnostic and therapeutic advances in high-income countries have enabled first complete remission rates to exceed 80% and induction-related mortality (IRM) rates to drop below 5%. In contrast, IRM in patients with ALL from low- and middle-income countries (LMICs) remains relatively high owing to a lack of infrastructure and resources limiting accessibility to molecular diagnosis, pediatric-inspired treatment regimens, and supportive measures during the post-induction period. In addition, the clinical management for adolescents and young adults (AYAs) with ALL is particularly challenging due to their transition from pediatric to adult care and their increased likelihood of presenting with high-risk ALL. Compared with children, AYAs also present with a greater incidence of Philadelphia chromosome positivity (Ph+), Philadelphia-like, and BCL2/MYC rearrangements, and have lower 5-year overall survival and increased mortality in first complete remission.
The ALL Hub has previously reported on comparison of outcomes in AYA versus younger patients with high-risk B-cell ALL and produced an educational theme article outlining the gene expression profiles in AYA and adult patients which can be found here. Below, we summarize the key findings by Inclan-Alarcon, et al., published in Blood Research, investigating the risk factors associated with IRM in AYAs and adults with ALL treated in LMIC.1
Study design
This was a retrospective cohort study comprising patients aged ≥16 years with newly diagnosed ALL according to the 2016 World Health Organization (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissues between 2009 and 2016 in Mexico. Patients were classified as AYAs if their age was ≤40 years.
- A high-risk cytogenetic profile was defined as the presence of the translocation t(9,22) (Ph+), mixed lineage leukemia 1 rearrangements, hypodiploidy, and/or complex karyotypes.
- Central nervous system (CNS) status was defined as CNS-1, -2, or -3 according to the National Comprehensive Cancer Network.
- Socioeconomic status was defined by monthly household income:
- Low – <180.00 US dollars
- Middle – ≥180.00 US dollars
- High – Patients with private health insurance
- IRM was defined as death within 60 days after initiation of induction regimen, during post-chemotherapy, and not associated with refractoriness or progression.
- Cause of death was defined as either:
- Infectious – if directly associated with septic shock or other complications related to infection
- Hematologic – if attributed to major hemorrhagic or thrombotic events
- Other – none of the above
Results
Baseline characteristics
A total of 167 patients were included of which 67.1% were AYAs and 32.9% were adults. The median age was 28 years (range, 16–70 years) and 50.9% of patients were male. Overall, 96.4% of patients presented with an Eastern Cooperative Oncology Group Performance Status ≤2 and 48.5% patients had one or more additional comorbidities at diagnosis (Table 1).
The median follow-up was 10 months (range, 10–108 months) and 77.2% of all patients achieved first complete remission. With regards to induction therapy, 65.3%, 28.7%, and 6% of patients were administered hyper-CVAD, HOP0195/0612 (institutional protocol), and other regimens, respectively.
Table 1. Baseline characteristics*
|
ALL, acute lymphoblastic leukemia; AYA, adolescent and young adults; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; MLL, mixed-lineage leukemia; Ph, Philadelphia; SES, socioeconomic status. |
||
|
Characteristics, % (unless stated otherwise) |
AYA |
Adults |
|---|---|---|
|
Male |
52.7 |
47.3 |
|
Median age (range), years |
22 (16–39) |
51 (40–70) |
|
SES |
||
|
Low |
99.1 |
85.5 |
|
Middle |
0 |
5.4 |
|
High |
0.9 |
9.1 |
|
ECOG Performance Status ≤2 |
97.3 |
94.5 |
|
ALL phenotype |
||
|
B-cell leukemia |
97.3 |
98.2 |
|
Pre-B |
93.6 |
100 |
|
Mature B |
5.5 |
0 |
|
Pro-B |
0.9 |
0 |
|
T-cell leukemia |
2.7 |
1.8 |
|
Comorbidities |
40.5 |
65.5 |
|
Diabetes mellitus |
1.8 |
27.3 |
|
Hypertension |
3.6 |
16.4 |
|
Obesity |
18.8 |
27.3 |
|
Cytogenetic abnormalities† |
25.5 |
38.5 |
|
Ph chromosome |
15.5 |
23.1 |
|
MLL rearrangement |
0.9 |
1.9§ |
|
Hypodiploidy |
0 |
3.9 |
|
Complex karyotype |
4.6‡ |
1.9 |
|
Others |
7.3 |
9.6 |
|
CNS involvement |
11.6 |
10.9 |
A total of 91% patients reported induction-related complications; infections and metabolic were the most common types (Figure 1).
Bacterial isolate was obtained in 69.9% of patients with infectious complications while 19.9% of patients showed invasive fungal infections. In total, 46 patients developed shock during induction therapy; 39 (84.8%) of cases were due to sepsis.
IRM was reported in 12% (n = 20) of patients, and the cause of death was infectious and hemorrhagic complication in 77.8% and 16.7% of these patients, respectively. The IRM in AYAs compared to adults was 11.6% vs 12.7% (p = 0.83) with no difference in the cause of death.
Figure 1. Induction-related complications*
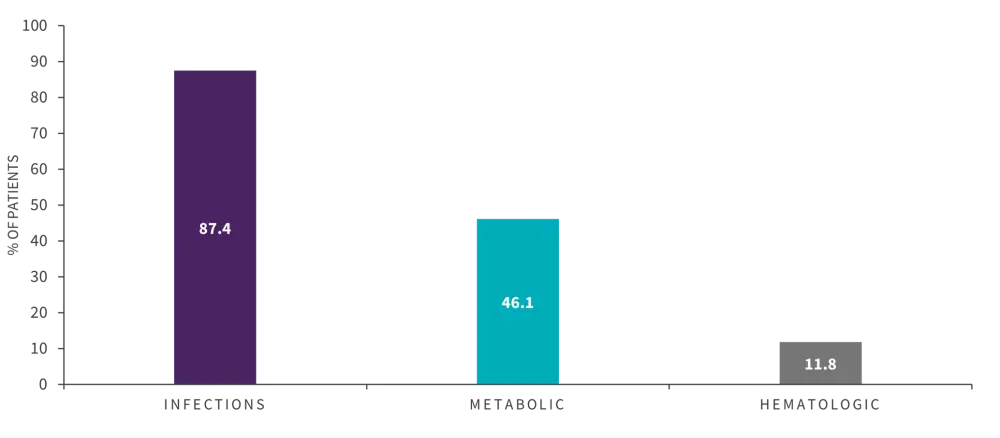
*Adapted from Inclan-Alarcon, et al.1
In univariate analysis the following factors were associated with decreased OS (Table 2):
- CNS status at diagnosis
- tumor lysis syndrome
- disseminated intravascular coagulation
- shock
- bloodstream infection
- dialysis requirement
- assisted mechanical ventilation requirement
In multivariate analysis, CNS-2 status (p = 0.001) and dialysis requirement (p = 0.001) remained significant associated with an increased IRM (Table 2).
Table 2. Factors associated with decreased OS after induction*
|
CNS, central nervous system; CI, confidence interval; DIC, disseminated intravascular coagulation; HR, hazard ratio; OS, overall survival; TLS, tumor lysis syndrome. |
||||
|
|
Univariate analysis |
Multivariate analysis |
||
|
Factors, % (unless otherwise stated) |
OS Day +60 |
p value |
HR (95% CI) |
p value |
|---|---|---|---|---|
|
CNS-3 vs CNS-2 vs CNS-1 |
73.7 vs 37.5 vs 92.7 |
<0.001 |
— |
— |
|
CNS-2 |
— |
— |
10.10 (2.67–38.18) |
0.001 |
|
CNS-3 |
— |
— |
3.078 (0.81–11.67) |
0.103 |
|
TLS |
75.3 vs 85.12 |
0.005 |
— |
|
|
DIC |
66.7 vs 89.4 |
0.037 |
— |
|
|
Shock |
63.2 vs 93.8 |
<0.001 |
— |
|
|
Bloodstream infection |
83.1 vs 95.3 |
0.020 |
— |
|
|
Dialysis requirement |
28.6 vs 91.0 |
<0.001 |
9.15 (2.44–34.34) |
0.001 |
Conclusion
This retrospective study was the first to examine IRM and associated risk factors in AYA and adult populations from a LMIC setting. The study demonstrated that AYA and adult patients with ALL treated with intensive induction regimens had high IRM (12%) that was attributed to infection in most instances (87.4%). Dialysis requirement (indicative of end-stage organ disease) and CNS status were independently associated with increased IRM in this population. Although not extensively studied, association between CNS status and IRM may be attributed to greater disease burden and proliferation of aggressive leukemic phenotypes.
This study was limited by its retrospective nature, small sample size, and lack of molecular profile assessment. Further research is needed to investigate IRM and the associated factors utilizing prospective study design that include pediatric-appropriate regimens and molecular diagnoses.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content

