All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Consolidation therapy in newly diagnosed adult patients with Ph-negative B-cell ALL
Featured:
Do you know... The U.S. Food and Drug Administration (FDA) has approved blinatumomab as a consolidation therapy for which of the following indications?
During the ALL Hub Steering Committee meeting, Mark Litzow chaired a discussion on consolidation therapy in newly diagnosed (ND) adult patients with Philadelphia chromosome-negative (Ph−) B-cell acute lymphoblastic leukemia (B-ALL), featuring Oliver Ottmann, Wendy Stock, André Baruchel, Sabina Chiaretti, Nicolas Boissel, José María Ribera, and Charles Mullighan.
Consolidation therapy in newly diagnosed adult patients with Ph-negative B-cell ALL
Litzow provides an overview of the phase III ECOG-ACRIN E1910 trial (NCT02003222) assessing the safety and efficacy of consolidation chemotherapy with or without blinatumomab in patients aged 30–70 years with ND Ph− B-ALL in measurable residual disease (MRD)-negative remission (Figure 1).
Figure 1. ECOG-ACRIN E1910 study design*
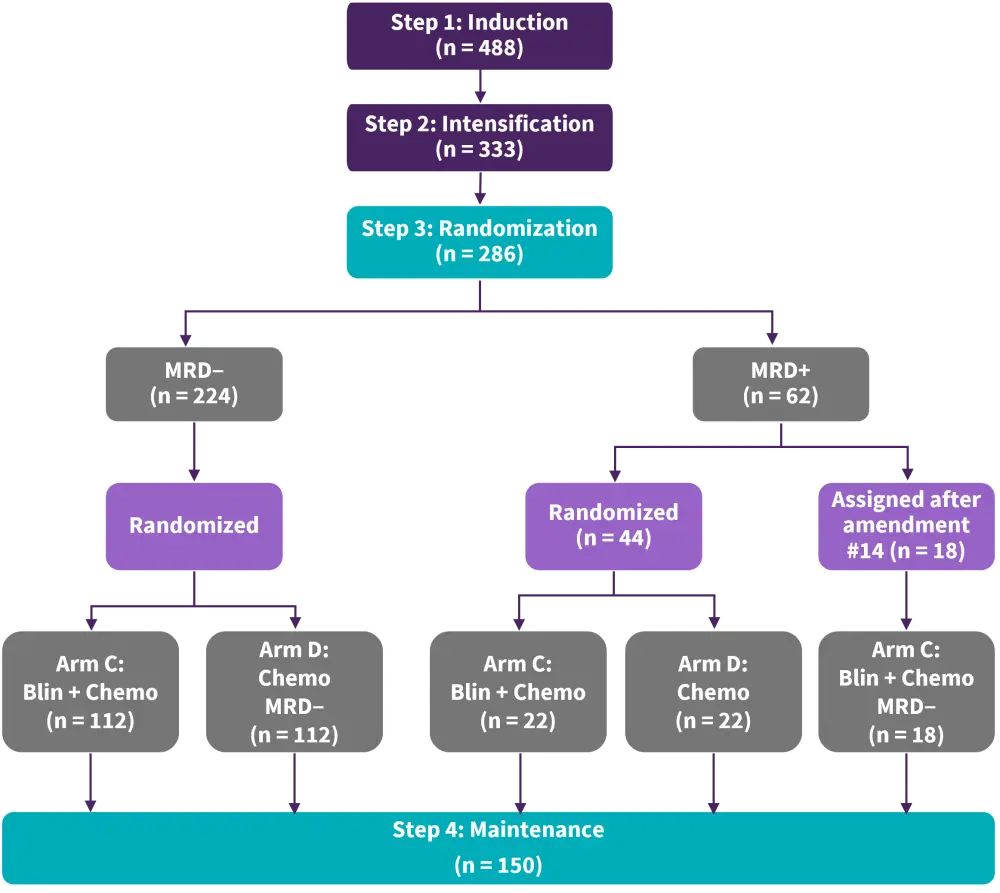
Blin, blinatumomab; chemo, chemotherapy; MRD, measurable residual disease.
*Adapted from Litzow MR, et al.1
The primary endpoint was met; after a median follow-up of 43 months, the 3-year OS rate was 85% in the blinatumomab plus chemotherapy arm vs 68% in the chemotherapy alone arm (Figure 2).1
Figure 2. OS comparison: MRD-negative patients*
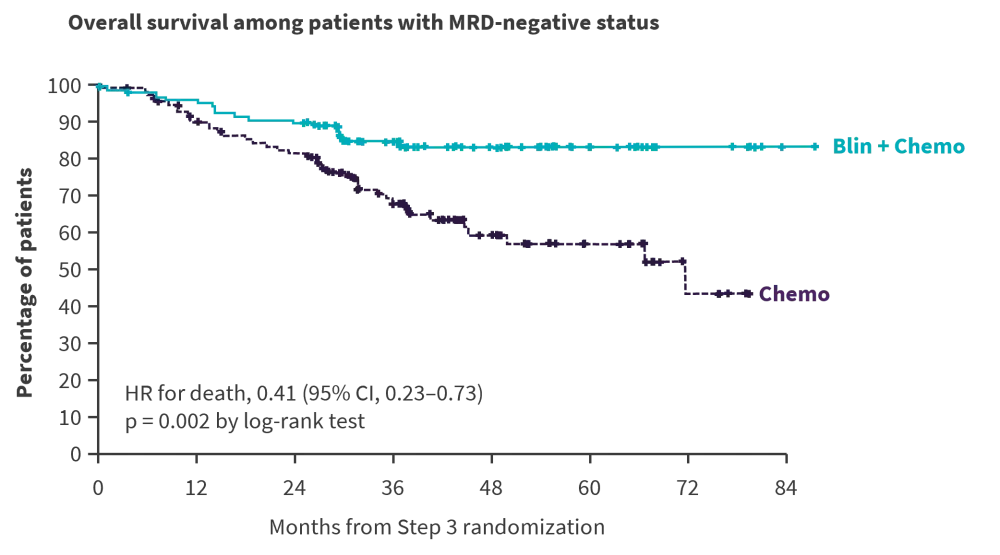
Blin, blinatumomab; Chemo, chemotherapy; CI, confidence interval; HR, hazard ratio; MRD, measurable residual disease; NRM, non-relapse mortality; OS, overall survival.
*Adapted from Litzow MR, et al.1
This survival benefit was also observed in patients aged <55 years (hazard ratio [HR], 0.16; 95% confidence interval [CI], 0.05–0.47; p <0.001).1 The survival benefit of blinatumomab in patients aged ≥55 years did not reach statistical significance (HR, 0.66; 95% CI, 0.33–1.35; p = not significant).1 The survival benefit was also assessed across risk groups, with a significant improvement in patients with favorable (HR, 0.00; 95% CI, 0.00–not applicable; p = not applicable) and unfavorable (HR, 0.39; 95% CI, 0.19–0.78; p = 0.008) molecular risk.1 Based on results from this trial, and other trials, the U.S. Food and Drug Administration (FDA) approved blinatumomab as a consolidation therapy for adult and pediatric patients with CD19+ Ph− B-ALL, irrespective of MRD status.2
Discussion
Key discussion points included:
- The panel commented that the use of immunotherapy in earlier lines of treatment could be beneficial and reduce the amount of chemotherapy used.
- The optimal number of cycles of blinatumomab was discussed, and four cycles was recommended as the standard approach.
- MRD analysis in the ECOG-ACRIN E1910 trial was discussed; next-generation sequencing MRD analysis will be carried out to assess the impact of MRD beyond the 10−4 threshold.
- The impact of age and clinical features on outcomes was highlighted as an area for further exploration.
- Finally, the panel highlighted that full genomic characterization for the ECOG-ACRIN E1910 trial is ongoing.
Listen to this discussion as a podcast:
Consolidation therapy in newly diagnosed adult patients with Ph-negative B-cell ALL
Your opinion matters
I commit to reviewing the latest clinical data and recommendations for the optimal use of blinatumomab in patients with Ph-negative B-ALL.
This educational resource is independently supported by Amgen. All content is developed by SES in collaboration with an expert steering committee; funders are allowed no influence on the content of this resource.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content


 Charles Mullighan
Charles Mullighan José María Ribera
José María Ribera Wendy Stock
Wendy Stock Sabina Chiaretti
Sabina Chiaretti Oliver Ottmann
Oliver Ottmann Mark Litzow
Mark Litzow André Baruchel
André Baruchel Nicolas Boissel
Nicolas Boissel

