All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Educational theme | Sequencing and combining immunotherapies in ALL
Do you know... Which of the following novel therapies is most suitable for the treatment of patient with MRD-positive relapsed/refractory ALL?
Overall, historical use of salvage chemotherapy has yielded poor outcomes in relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL).1 Over the past decade, introduction of the highly effective and safe novel antigen-targeted immunotherapies, blinatumomab, inotuzumab ozogamicin (InO), and CD19-targeted chimeric antigen receptor (CAR) T‑cell therapies, have significantly advanced the treatment landscape of R/R ALL.1,2
With the availability of all these agents and eligibility of most in adult R/R B-cell ALL (B-ALL), the optimal therapy choice varies according to different clinical settings. Additionally, treatment sequencing and combinations are important strategies to further improve clinical outcomes. The ALL Hub previously published an expert discussion on treatment sequencing in R/R B-ALL.
In this educational theme article, we review the current landscape of antigen-targeted therapies and discuss practical considerations and recommendations for the optimal selection, sequencing, and combined use according to different clinical settings using data published by Aldoss et al.1 in The American Journal of Hematology and data published by Kegyes et al.2 in Blood Reviews.
Treatment landscape of FDA-approved novel immunotherapies in B-ALL1
Approved by the U.S. Food and Drug Administration (FDA), blinatumomab is an anti-CD3/CD19 bispecific T‑cell engager for R/R ALL with persistent measurable residual disease (MRD). Its approval was based on results from the phase III TOWER trial (NCT02013167), which demonstrated higher complete remission (CR) rates (44% vs 25%) and longer median overall survival (OS; 7.7 months vs 4 months) compared with salvage chemotherapy. The treatment indication of blinatumomab for patients with persistent MRD was based on results from the phase II BLAST trial (NCT01207388), which reported an 80% MRD conversion rate, progression to allogeneic hematopoietic cell transplant (allo-HCT) in 67% of patients, and a median OS of 36.5 months.
InO is an FDA-approved anti-CD22 monoclonal antibody for the treatment of R/R B-cell ALL. InO achieved higher CR (81% vs 29%) and MRD rates (78% vs 28%), and also facilitated a higher frequency of allo-HCT consolidation (41% vs 11%), when compared with salvage chemotherapy in the phase III INO-VATE trial (NCT01564784).
Two CD19 autologous CAR T-cell products, tisagenlecleucel (tisa-cel) and brexucabtagene autoleucel (brexu-cel), have been approved by the FDA for R/R B-ALL based on results from the ELIANA (NCT02435849) and ZUMA-3 (NCT02614066) trials, respectively. Tisa-cel is indicated for patients aged ≤25 years with R/R B-ALL following two prior lines of therapies or relapsed disease after allo‑HCT. The ELIANA trial reported a CR/CR with incomplete count recovery (CRi) rate of 81%, a 95% MRD-negativity rate among responders, and 5-year relapse-free survival and OS rates of 42% and 55%, respectively. Brexu-cel is approved for adults (aged ≥18 years) with R/R B-ALL after two lines of prior therapies or relapsed disease after allo-HCT. In the ZUMA-3 trial, brexu-cel yielded a CR/CRi rate of 71% and a MRD-negativity rate of 76% in responders.
Practical considerations for novel immunotherapies in disease management1
Practical considerations for the selection, sequencing, and combination of these novel immunotherapies (blinatumomab, InO, and CD19 CAR T cells) include, but are not limited to, disease burden, extramedullary disease (EMD), and safety profile (Figure 1). The advantages and limitations of each therapy are summarized in Figure 2.
Figure 1. A comparison of the considerations required for approved novel immunotherapies in R/R B-ALL*
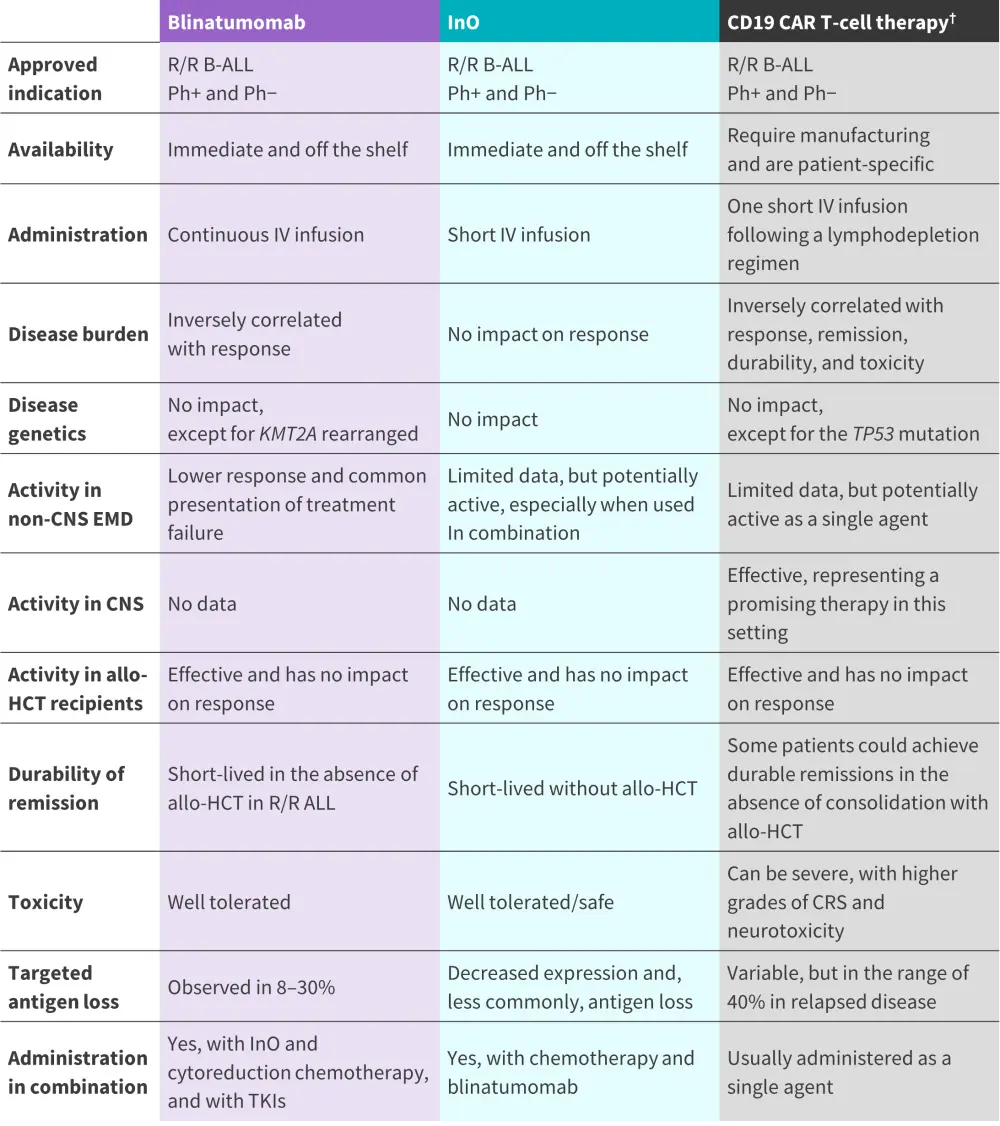
Allo-HCT, allogeneic hematopoietic cell transplant; B-ALL, B-cell acute lymphoblastic leukemia; CAR, chimeric antigen receptor; CNS, central nervous system; InO, inotuzumab ozogamicin; IV, intravenous; Ph, Philadelphia chromosome; TKI, tyrosine kinase inhibitor.
*Adapted from Aldoss, et al.1
†Includes both FDA-approved agents in R/R B-ALL; brexucabtagene autoleucel and tisagenlecleucel.
Figure 2. Advantages and limitations of approved novel immunotherapies in R/R B-ALL*
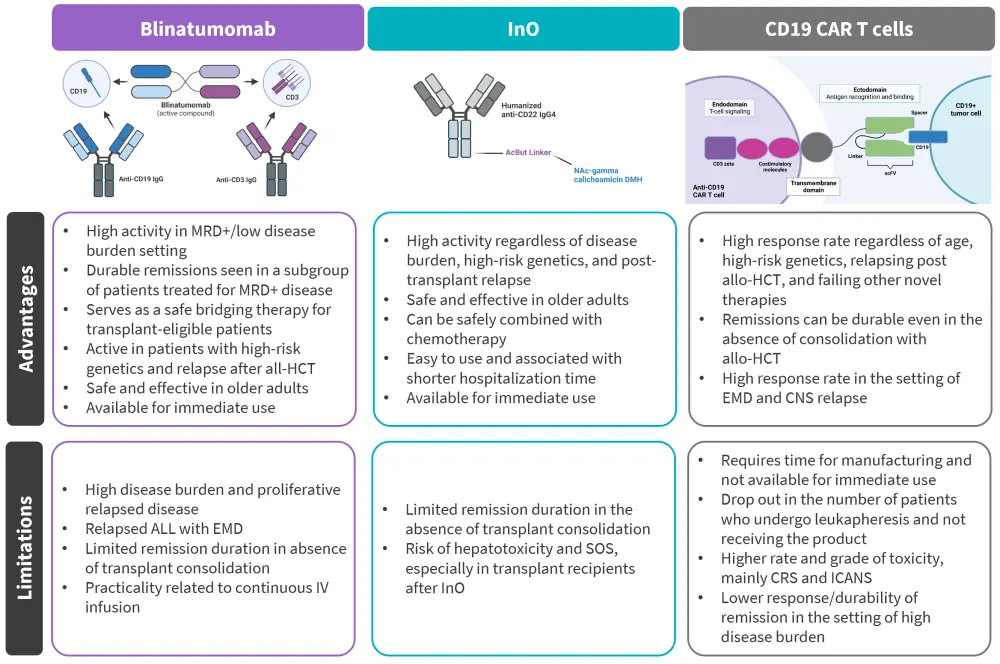
allo-HCT, allogeneic hematopoietic cell transplant; B-ALL, B-cell acute lymphoblastic leukemia; CAR, chimeric antigen receptor; CNS, central nervous system; CRS, cytokine release syndrome; EMD, extramedullary disease; ICANS, immune cell-associated neurotoxicity syndrome; InO, inotuzumab ozogamicin; MRD, measurable residual disease; SOS, sinusoidal obstruction syndrome.
*Adapted from Aldoss, et al.1 Created using BioRender.com.
Blinatumomab
Disease burden
Studies have shown an association between pretreatment disease burden and responses to blinatumomab, with higher response rates observed in patients with lower disease burden, especially in those with MRD-positivity or <50% bone marrow (BM) blasts, compared with those with high disease burden, defined as ≥50% BM blasts (CR/CRi rate, 66–73% vs 29–34%, respectively). As such, blinatumomab is a feasible option for patients with low disease burden, but not recommended for patients with high marrow blasts/or proliferative disease.
Extramedullary disease
Inferior responses have been reported in patients with a history or evidence of active EMD at the time of blinatumomab therapy. In one retrospective analysis of adults with R/R ALL, 36% of patients who were refractory to blinatumomab and 50% who initially responded and later relapsed had EMD. Moreover, EMD with central nervous system (CNS) involvement is observed more often in patients responding to blinatumomab at the time of relapse; thus, single-agent blinatumomab is not recommended or recommended with cautionary use in patients with EMD at relapse.
Remission durability
Blinatumomab followed by allo-HCT consolidation over single-agent blinatumomab is the preferred option for adults with R/R ALL given the higher durable remission rates. A subset of patients with persistent MRD treated with blinatumomab without allo-HCT attained durable remissions; therefore, it may be an option for patients who are not eligible for allo-HCT.
Safety
Blinatumomab has a favorable safety profile, making it a practical option for older or frail patients, as well as patients relapsing post allo-HCT. Its toxicities include cytokine release syndrome and neurotoxicity, which are usually of low-grade and reversible with corticosteroids or drug discontinuation.
Practicality
Although its standard administrative use involving continuous intravenous (IV) infusion raises practicality concerns, preliminary results on its subcutaneous use are encouraging and remain a promising alternative.
Inotuzumab ozogamicin
Disease burden
Pretreatment disease burden does not significantly impact InO response, with studies showing comparable CR rates in patients with <50% vs ≥50% pretreatment marrow blasts (87% vs 78%, respectively). InO could be a viable option for patients with high disease burden and could also serve as a feasible cytoreductive treatment prior to blinatumomab or CAR T-cell therapy in proliferative leukemia.
Extramedullary disease
Like blinatumomab, earlier use of InO is associated with higher outcomes; in a phase II study, higher response rates (76% vs 50%, respectively) were reported for first-line InO versus when it was administered in a subsequent setting. There are limited data on the activity of InO in non-CNS EMD, though some studies have shown its activity in ALL with EMD, without excessive risk of EMD relapse. A post hoc analysis of the INO-VATE study, which included patients with ALL and EMD, reported CR/CRi in 5/7 patients treated with InO vs 2/5 patients in the standard of care arm. Additionally, 4/59 vs 2/41 EMD relapses occurred in the InO vs standard of care arms, respectively. To date, there are no data on the activity of InO in CNS disease.
Practicality
The short IV infusion administration rate and the shorter hospitalization time compared with chemotherapy is an advantage of InO.
Remission durability
The durable remission rates of InO are short-lived without the use of allo-HCT consolidation. In one study, InO responders who received allo-HCT consolidation achieved a higher 2-year OS rate when compared with those without allo-HCT (39% vs 13%, respectively). Therefore, it can be considered as a bridging treatment to allo-HCT (or CAR T-cell therapy in cases of transplant-ineligible R/R ALL), although sinusoidal obstruction syndrome (SOS) should be monitored carefully.
Safety
Although InO is well tolerated, hepatoxicities and the risk of SOS with or without prior allo-HCT are safety concerns. Known risk factors of SOS in transplant include the use of dual-alkylator conditioning regimens, abnormally elevated pretransplant bilirubin level, history of liver disease, and more than two cycles of inotuzumab. Therefore, in these cases, preventative strategies such as avoiding double alkylators or limiting InO to two cycles should be considered.
CD19 CAR T-cell therapy
Disease burden
Like blinatumomab, disease burden is predictive of CD19 CAR T-cell therapy outcomes. High leukemia burden pre-lymphodepletion is linked to inferior outcomes and increased risk of CAR T-cell therapy-related toxicities. In the ZUMA-3 trial, high (76–100% marrow blasts) versus low (0-75% marrow blasts) disease burden among patients treated with brexu-cel resulted in lower CR rates (42% vs 80–91%, respectively). Therefore, brexu-cel could be used in low disease burden settings, such as persistent MRD, and could potentially replace allo-HCT as a consolidation approach in patients with R/R ALL who are eligible for transplant.
Extramedullary disease
Despite limited and conflicting data, CD19 CAR T-cell therapy has achieved response rates of 50–100% in patients with R/R ALL and EMD; therefore, it represents a promising option in this setting. CAR T-cell therapies have also demonstrated CNS penetrative activity. A study that included 48 patients with R/R ALL and CNS involvement treated with CD19 CAR T-cell therapy reported a remission rate of 85% and a 12-month CNS relapse incidence of 11%. This suggests CD19 CAR T-cell therapies are a promising option for R/R ALL with EMD and/or CNS involvement.
Remission durability
In a subset of patients, CD19 CAR T-cell therapy has yielded durable remission rates without allo‑HCT; however, this effect is likely impacted by the CAR T-cell construct and CAR T-cell persistence. For example, the ELIANA study reported a 3-year relapse-free survival rate of 76% for patients treated with tisa-cel alone. As such, this can be considered an option for patients ineligible for transplant or as first-salvage therapy for those with relapsed ALL post-allo-HCT. Several studies still support the use of allo-HCT consolidation post-CD19 CAR T-cell therapy, thus it is considered in patients eligible for transplant.
Safety
Compared with blinatumomab, CAR T-cell therapy-related toxicities can be severe, mainly including high-grade prolonged cytokine release syndrome and neurotoxicities. Infectious complications and prolonged cytopenias are other toxicities occurring with CAR T-cell therapy; however, these can be managed with supportive care strategies, such as prophylactic measures.
Practicality
The longer engineering and manufacturing time of CAR T-cell therapies can present practical issues and should be carefully considered, especially in cases of proliferative leukemia (R/R ALL).
Recommended immunotherapy for different clinical cases1
In Figure 3, we summarize the recommended immunotherapy depending on the clinical case.
Figure 3. Recommended immunotherapy according to clinical condition*
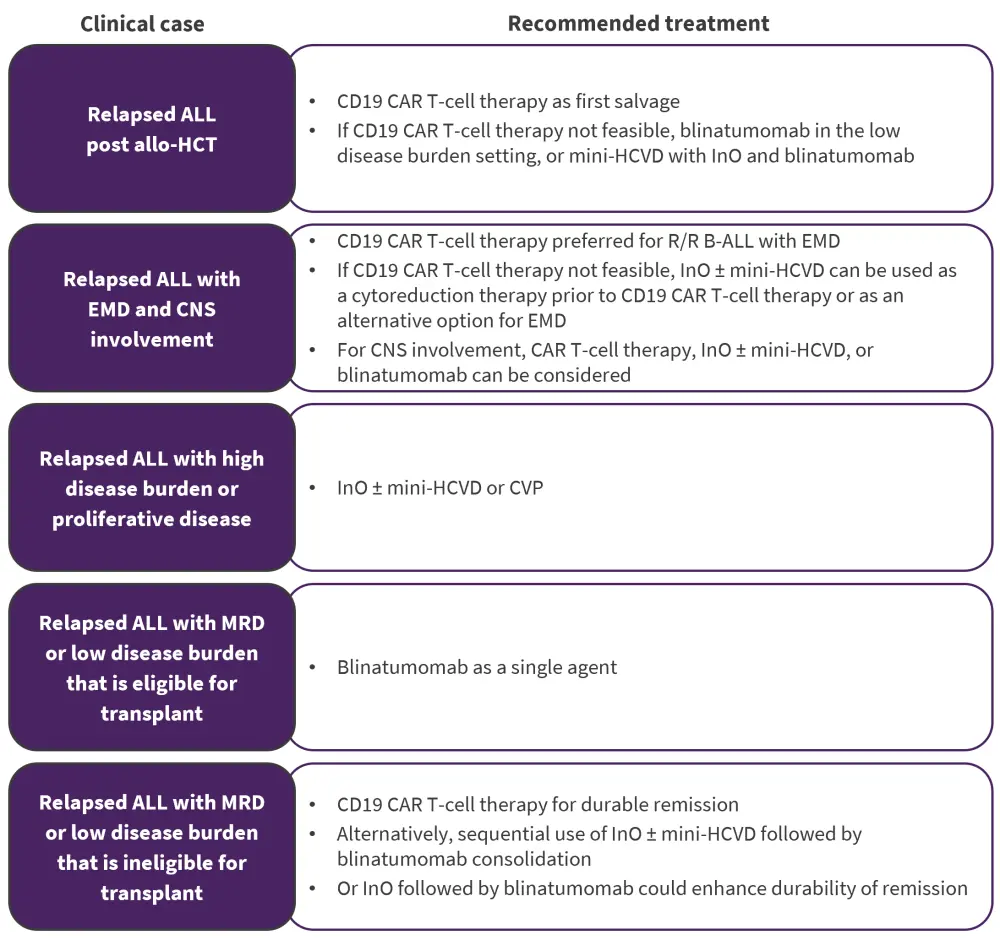
Allo-HCT, allogeneic hematopoietic cell transplant; B-ALL, B-cell acute lymphoblastic leukemia; CAR, chimeric antigen receptor; CNS, central nervous system; CVP, cyclophosphamide, vincristine, and prednisone; EMD, extramedullary disease; mini-HCVD, mini-hyperfractionated cyclophosphamide, vincristine, and dexamethasone; MRD, measurable residual disease.
*Data from Aldoss, et al.1
Sequencing and combination of novel therapies1,2
Sequential use of blinatumomab and InO have demonstrated efficacy and safety in a phase I study (NCT03739814).2 Real-world data have also shown comparable response rates for InO post-blinatumomab therapy and blinatumomab post-InO therapy (58% vs 52%, respectively).1
InO with mini-HCVD (mini-hyperfractionated cyclophosphamide, vincristine, and dexamethasone) in newly diagnosed and/or R/R ALL and InO combined with cyclophosphamide, vincristine, and prednisone (CVP) are safe combinations.1 Both combinations can induce remission in patients with high disease burden or proliferative disease. InO ± mini-HCVD can also be used as bridging therapy for disease control prior to lymphodepletion in CAR T-cell therapy or as consolidation to blinatumomab and/or allo-HCT.1
InO ± mini-HCVD followed by blinatumomab consolidation could be an option in a low disease burden setting or patients with transplant ineligible R/R ALL, as discussed above.1 Mini-hyperCVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone) with a reduced-dose inotuzumab is a salvage therapy often used in advanced B-ALL for patients with high disease burden or as a bridging therapy for disease control prior to lymphodepletion in CAR T-cell therapy.1,2
CD19 CAR T-cell therapy after prior blinatumomab has attained high response rates if CD19 expression is retained on the tumor blasts; however, recent analyses have reported inferior outcomes to CD19 CAR T-cell therapy for patient’s refractory to blinatumomab compared with blinatumomab-naïve and/or blinatumomab responders.1
Given the non-overlapping toxicities and activities of blinatumomab and InO, mini-HCVD with InO and blinatumomab is a combination regimen undergoing current investigation (NCT02877303).1 Among 96 patients with R/R B-ALL, the overall response rate was 80% and 91% in the total cohort and first salvage therapy cohort, respectively. The 3-year OS for the entire cohort and those in first salvage was 33% and 42%, respectively. Compared with single-agent InO, this combination regimen demonstrated a longer median OS (13 months vs 6 months); therefore, it represents a promising option for patients with R/R Philadelphia-negative ALL. A further reduced intensity of InO in this study led to a SOS rate reduction of 13% to 3%.1
There are ongoing studies of blinatumomab combined with AMG404, a PD-1 checkpoint inhibitor (NCT04524455), as well as with other PD1 inhibitors or CTLA-4 inhibitors, such as pembrolizumab, nivolumab, and ipilimumab (NCT03512405, NCT04546399, and NCT02879695, respectively).2 The phase III ECOG-ACRIN E1910 trial (NCT02003222), recently reported on the ALL Hub, investigated consolidation chemotherapy ± blinatumomab in patients with newly diagnosed Philadelphia-negative B-ALL, with blinatumomab + consolidation chemotherapy demonstrating better survival rates compared with chemotherapy alone.2
Conclusion
This review highlights the treatment landscape of the FDA-approved novel immunotherapies, blinatumomab, InO, and CAR T-cell therapy, in R/R B-ALL, including their optimal selection, sequencing, and combined use for disease management. Practical considerations for their use should include disease burden activity, presence of EMD, safety profile, as well as other patient, disease, and treatment-specific factors. Research to improve the clinical activity of these therapies, such as the concept of cytoreduction prior to inotuzumab, reducing toxicities in CAR T-cell therapy, and safety of therapies in EMD and CNS involvement, is needed. Further investigations on combination and sequencing therapies will likely further improve outcomes.
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



