All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit Know ALL.
The all Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the all Hub cannot guarantee the accuracy of translated content. The all and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
The ALL Hub is an independent medical education platform, sponsored by Amgen, Autolus, Jazz Pharmaceuticals, and Pfizer and supported through an educational grant from the Hippocrate Conference Institute, an association of the Servier Group. Funders are allowed no direct influence on our content. The levels of sponsorship listed are reflective of the amount of funding given. View funders.
Now you can support HCPs in making informed decisions for their patients
Your contribution helps us continuously deliver expertly curated content to HCPs worldwide. You will also have the opportunity to make a content suggestion for consideration and receive updates on the impact contributions are making to our content.
Find out more
Create an account and access these new features:
Bookmark content to read later
Select your specific areas of interest
View ALL content recommended for you
Ph-like ALL: An overview of the genomic landscape, epidemiology, diagnosis, and treatment
Do you know... Compared with non-Philadelphia chromosome-like cases, which of the following clinical characteristics are associated with Philadelphia chromosome-like acute lymphoblastic leukemia?
Philadelphia chromosome-like acute lymphoblastic leukemia (Ph-like ALL) is a high-risk subtype of B-cell ALL associated with poor clinical outcomes, including high rates of induction failure, minimal residual disease (MRD) persistence, and relapse. It is characterized by a similar gene expression profile to Philadelphia-positive ALL, but lacks the BCR-ABL1 fusion gene.1 Although Ph-like ALL can occur at any age, it most frequently occurs in adolescents and adults.2
In this article, we provide an overview of the epidemiology, clinical characteristics, genomic landscape, diagnosis, and treatment strategies of Ph-like ALL.
Epidemiology and clinical characteristics3,4
The epidemiology of Ph-like ALL is shown in Figure 1.
Figure 1. Epidemiology of Ph-like ALL*
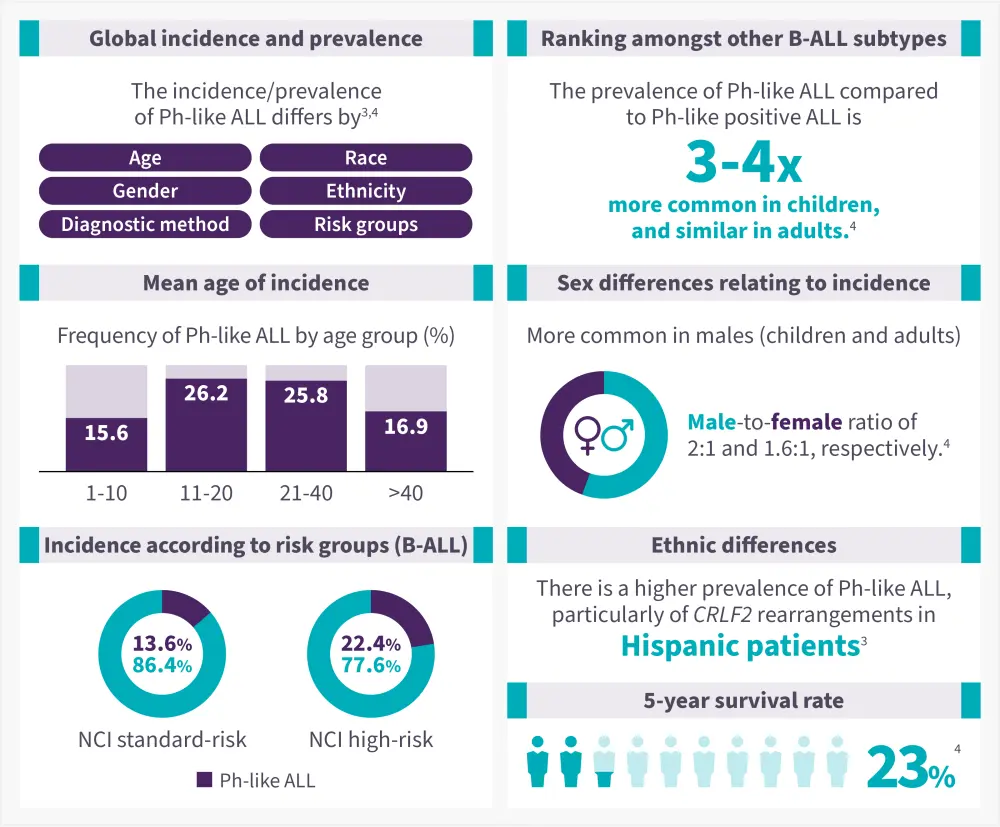
B-ALL, B-cell acute lymphoblastic leukemia; NCI, national cancer institute; Ph-like, Philadelphia-chromosome like.
Data from Kotb, et al.3; Pui, et al.4
Compared with non-Ph-like ALL, patients with Ph-like ALL have2,3:
- A higher leukocyte count (106,000 vs 59,000 per cubic millimeter; p < 0.001) at diagnosis
- A significantly lower 5-year overall survival rate (23% vs 59%; p = 0.006)
- Increased minimal residual disease levels after remission induction, lower continuous remission rates, and higher relapses
- Increased risk of treatment failure/induction failure
Genomic landscape
Ph-like ALL is a genetically heterogenous disease involving a spectrum of genetic alterations, activating cytokine receptor genes and kinase-signaling pathways; these are categorized into four genomically-defined subsets based on the similarity of functional fusion partners and underlying kinase-activating lesions (Table 1):
- JAK/STAT pathway gene alterations;
- ABL class alterations;
- Ras pathway mutations; and
- rare kinase fusions.
Table 1. Genomic landscape of Ph-like ALL kinase rearrangements, therapeutic targets, and clinical trials*
| 3’ kinase gene | 5’ fusion partner genes | Kinase inhibitors | Clinical trials |
| JAK/STAT pathway alterations |
|||
| CRLF2 | CSF2RA, IGH, P2RY8 | Ruxolitinib | NCT024207175 (terminated) |
| JAK2 | ATF7IP, BCR, EBF1, ETV6, GOLGA5, HMBOX1, OFD1, PAX5, PCM1, PPFIBP1, RFX3, SMU1, SNX29, SSBP2, STRN3, TERF2, TPR, USP25, ZBTB46, ZNF274, ZNF340 | Ruxolitinib | NCT027239946 |
| EPOR | IGH, IGK, IGL, LAIR1, THADA | Ruxolitinib | NCT031177517 |
| TSLP | IQGAP2 | Ruxolitinib | — |
| IL2RB | MYH9 | Ruxolitinib | NCT035713218 |
| ABL class alterations |
|||
| ABL1 | CENPC, ETV6, FOXP1, LSM14A, NUP153, NUP214, RANBP2, RCSD1, SFPQ, SHIP1, SNX1, SNX2, SPTNA1, ZMIZ1 | Dasatinib, imatinib, others | NCT028830499 |
| ABL2 | PAG1, RCSD1, ZC3HAV1 | Dasatinib, imatinib | NCT0214341410 |
| CSF1R | MEF2D, SSBP2, TBL1XR1 | Dasatinib, imatinib | NCT024207175 |
|
PDGFRA |
FIP1L1 | Dasatinib, imatinib | NCT0300714711 |
|
PDGFRB |
ATF7IP, EBF1, ETV6, NUMA1, SNX29, SSBP2, TERF2, TNIP1, ZEB2, ZMYND8, ZNF608 | Dasatinib, imatinib | NCT031177517 |
| LYN | GATAD2A, NCOR1 | Dasatinib, imatinib | — |
| Other kinases |
|||
| NTRK3 | ETV6 |
Entrectinib Larotrectinib |
NCT0383496113 |
| PTK2B | KDM6A, STAG2, TMEM2 | FAK inhibitors | — |
| FGFR1 | BCR | Ponatinib | — |
| FLT3 | ZMYM2 | FLT3 inhibitors | — |
| TYK2 | MYB, SMARCA4, ZNF340 | JAK1/3 inhibitor | — |
| BLNK | DNTT | — | — |
| CBL | KANK1 | — | — |
| DGKH | ZFAND3 | — | — |
| ALL, acute lymphoblastic leukemia; FAK, focal adhesion kinase; JAK, Janus kinase; Ph-like, Philadelphia chromosome-like. *Adapted from Tran and Tasian.1 |
|||
Figure 2. Prevalence of Ph-like ALL alterations in A children, B adolescents, and C young adults*
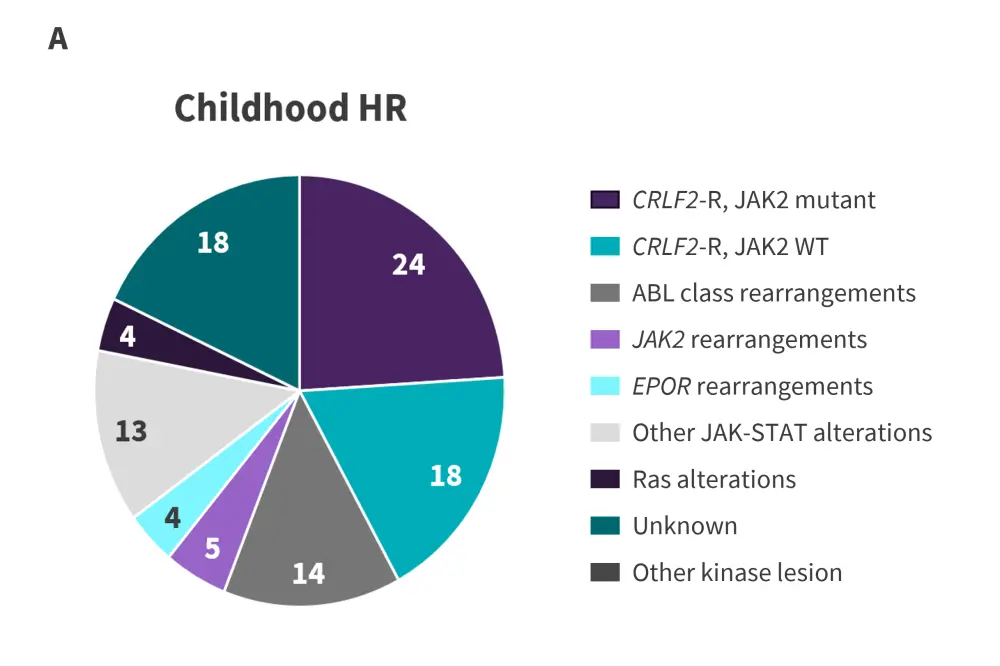
CRLF2-R, CRLF2-rearranged; HR, high risk; Ph-like ALL, Philadelphia chromosome-like acute lymphoblastic leukemia; WT, wild-type.
*Adapted from Tran and Loh.2
Figure 2. Prevalence of Ph-like ALL alterations in A children, B adolescents, and C young adults*
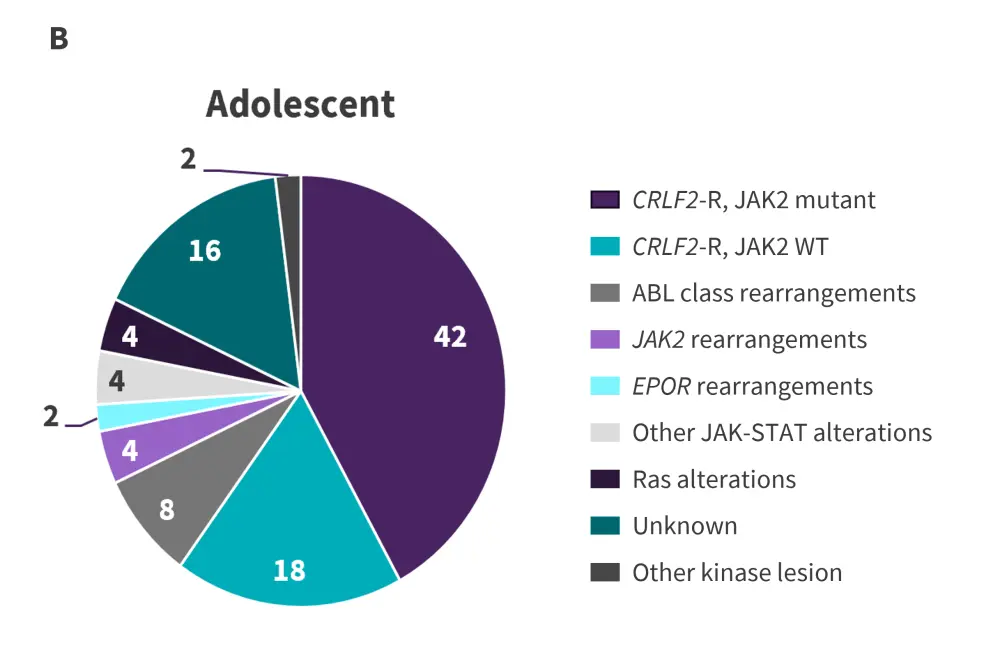
CRLF2-R, CRLF2-rearranged; HR, high risk; Ph-like ALL, Philadelphia chromosome-like acute lymphoblastic leukemia; WT, wild-type.
*Adapted from Tran and Loh.2
Figure 2. Prevalence of Ph-like ALL alterations in A children, B adolescents, and C young adults*
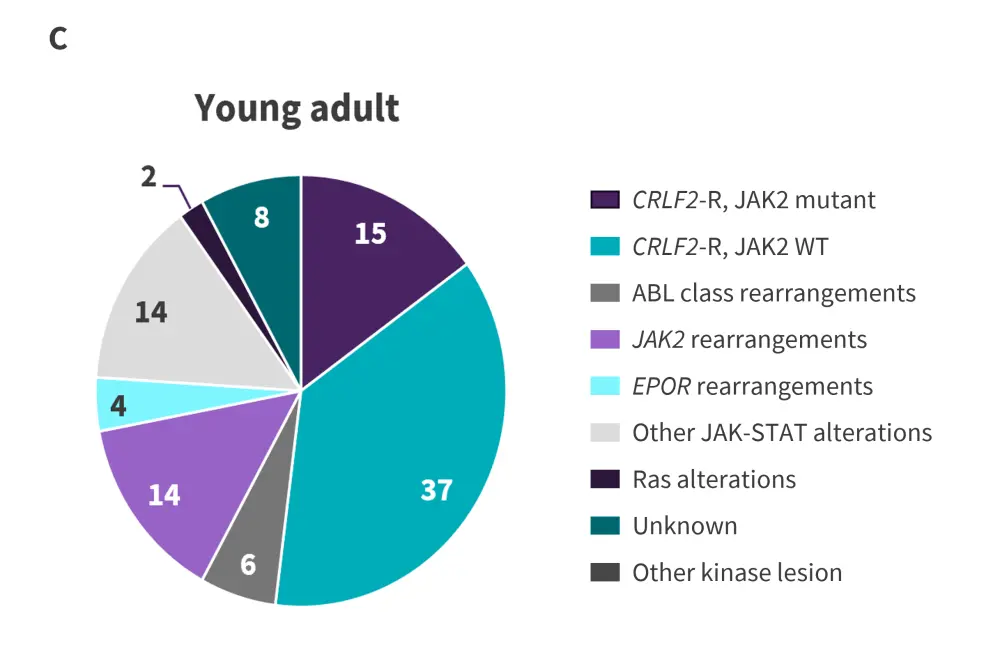
CRLF2-R, CRLF2-rearranged; HR, high risk; Ph-like ALL, Philadelphia chromosome-like acute lymphoblastic leukemia; WT, wild-type.
*Adapted from Tran and Loh.2
Key genomic alterations in Ph-like ALL are summarized in Figure 3.
Figure 3. Genomic landscape of alterations in Ph-like ALL*
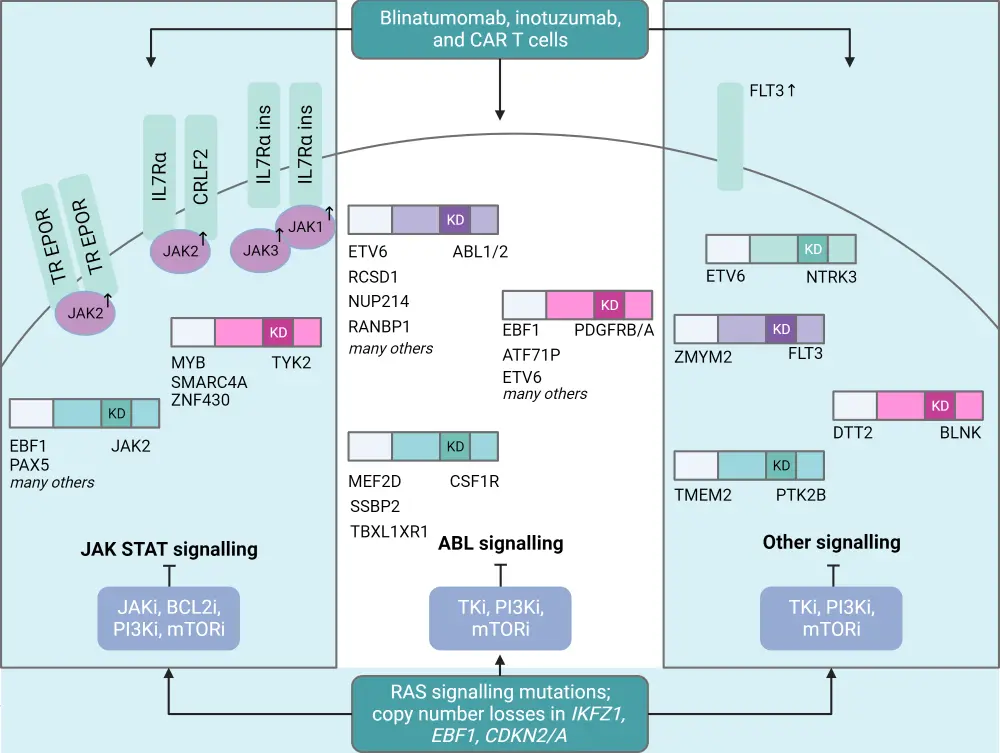
CRLF2-R, CRLF2-rearranged; HR, high risk; Ph-like ALL, Philadelphia chromosome-like acute lymphoblastic leukemia; WT, wild-type.
*Adapted from Tran and Loh.2
Diagnosis
Given the genetic heterogeneity of Ph-like ALL, clinical diagnosis remains challenging; there is currently no standardized approach.1,15 The diagnostic approach of Ph-like ALL relies on a combination of gene expression profiling, reverse transcription polymerase chain reaction, fluorescence in situ hybridization, immunophenotyping, and DNA sequencing. Performing clinical diagnostics has predictive and prognostic implications, facilitating better risk stratification and personalized treatment approaches for patients with Ph-like ALL.3
A practical, cost-effective, and time-efficient clinical algorithm to screen the Ph-like gene expression profile among all newly diagnosed patients with high-risk B-ALL was suggested by Harvey and Tasian 16 (Figure 4).
Figure 4. Clinical diagnostic algorithm of high-risk B-ALL*
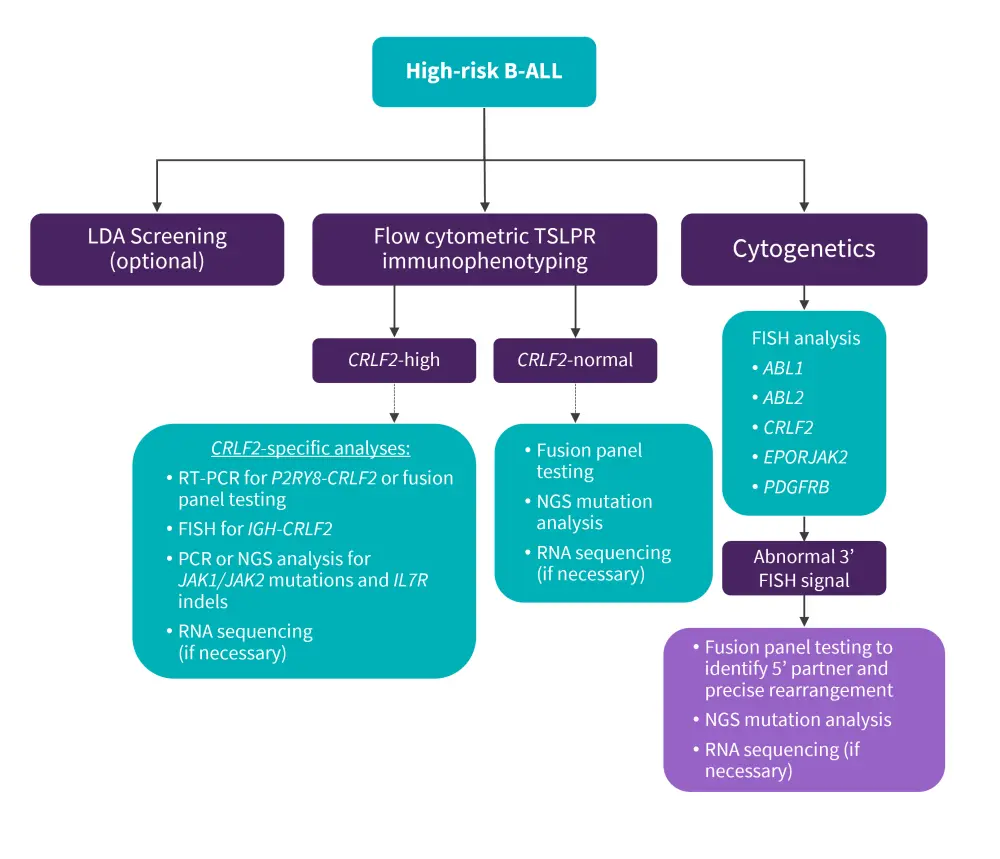
B-ALL, B-cell acute lymphoblastic leukemia; FISH, fluorescence in situ hybridization; LDA, low-density microarray; NGS, next-generation sequencing; PCR, polymerase chain reaction; RNA, ribonucleic acid; RT-PCR, reverse-transcriptase PCR; PDGFRB, platelet-derived growth factor receptor B; TSLPR, thymic stromal lymphopoietin receptor.
*Adapted from Harvey and Tasian.16
Management
To date, there is no standard treatment for patients with Ph-like ALL. Given the lack of guidelines, referral to clinical studies is recommended for newly diagnosed cases.17 If there are no studies available, the patients age and pre-existing comorbidities are considered when selecting appropriate therapies18
- Young patients, aged <40 years, are generally treated with pediatric-inspired regimens, such as C10403 or a modified Berlin-Frankfurt-Munich protocol.
- Given the poor outcomes with conventional chemotherapy in older patients, enrolment in frontline clinical studies, particularly those incorporating novel agents, is strongly encouraged.
- If no studies are available, a modified adult-based regimen focused on reducing treatment related toxicities to allow the early introduction of salvage therapy with novel agents.
- Across all ages, an earlier switch from chemotherapy to novel agents is recommended for poor responders or those with persistent MRD.
Tyrosine kinase inhibitors
Given that most cases of Ph-like ALL involve genetic alterations in kinases and/or cytokine receptors, the application of tyrosine kinase inhibitors (TKIs) has become an area of active research. In preclinical studies, TKIs have shown promising outcomes in patients with Ph-like ALL harboring either ABL-class fusions and JAK-STAT activating alterations. These encouraging results have led to a number of further clinical trials examining these novel agents in combination with established chemotherapy regimens.14,18
In addition, numerous single case reports exist detailing the benefits of the TKIs dasatinib and imatinib for patients with Ph-like ALL with ABL1 class fusions, either for slow responders, as salvage therapy for relapsed/refractory disease, or as preemptive maintenance therapy post-allogenic hematopoietic cell transplant in patients with persistent MRD.14,18 Among patients carrying the ABL-class fusion with a slow response on the UKALL2011 who were prospectively treated with imatinib, the 4-year relapse rate was 0% vs 62.5% and the event-free survival rate was 83.9% vs 37.5% for the TKI vs control groups, respectively.14
While several case reports have demonstrated clinical activity with ruxolitinib in combination with chemotherapy in JAK2-rearranged ALL, evidence for its single agent activity is largely unknown. The phase I part of the AALL1521 study (NCT02723994)6 demonstrated the efficacy and safety of ruxolitinib combined with chemotherapy in patients with B-cell ALL, harboring CRLF2, or JAK pathway alterations.18
Allogeneic stem cell transplantation
Allo-HSCT is a well-established for preventing relapse and is recommended as consolidation therapy for adults with high-risk ALL.19 An MRD-oriented approach, which was commenced in the GIMEMA LAL1913 study (NCT02067143)20, demonstrated that more patients with Ph-like ALL were allocated to transplant compared with patients with non-Ph-like ALL (53% vs 20%, respectively).18 Allo-HSCT is suggested for adults with Ph-like ALL who have persistent MRD post-consolidation, on an individual basis (considering age, chemotherapy resistance, and other risk factors).18
For older patients, transplant is considered as consolidation therapy based on donor availability, irrespective of MRD response.18 Although HSCT has shown promise in patients with Ph-positive ALL, its role in the treatment of Ph-like ALL is still unclear. El Fakih et al. suggested a treatment algorithm concerning when to consider Allo-HSCT in Ph-like ALL patients (Figure 5).20 Further studies are needed to fully elucidate the value of HSCT in this disease subset.19
Figure 5. Algorithm for the role of allogenic bone marrow transplant in patients with Ph-like ALL*
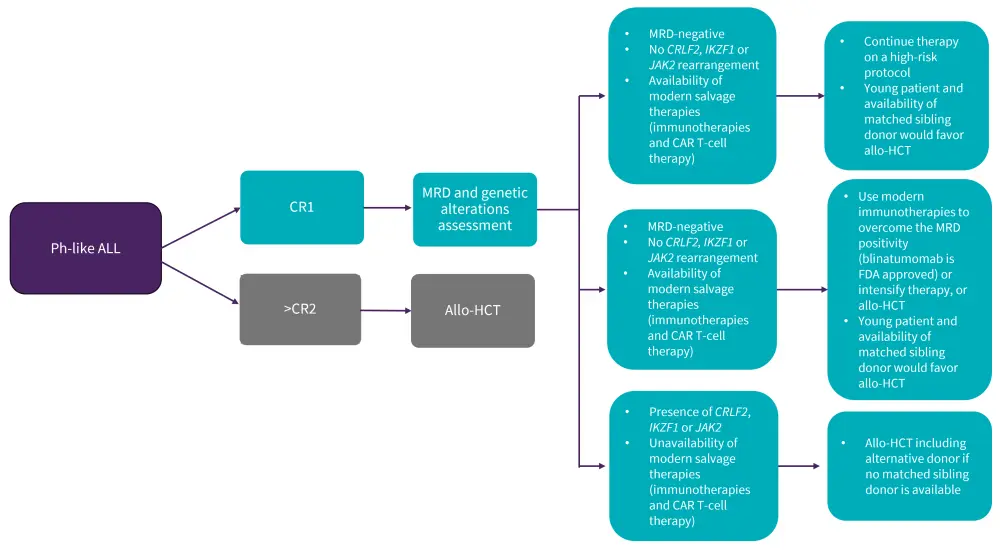
Allo-HCT, allogeneic hematopoietic stem cell transplantation; CAR T, chimeric antigen receptor T-cell; CR, complete remission; MRD, minimal residual disease; Ph-like ALL, Philadelphia chromosome-like acute lymphoblastic leukemia
*Adapted from Alghandour, et al.20
Targeted immunotherapies14,18
Considering the chemotherapy-resistant nature of Ph-like ALL, novel targeted immunotherapies have been investigated, such as those already approved for the treatment of B-ALL. Blinatumomab, a bispecific CD3/CD19 antibody, is currently approved by the U.S. Food and Drug Administration (FDA) for the treatment of B-cell precursor ALL. In adult patients with relapsed/refractory ALL, blinatumomab has demonstrated encouraging response rates, with a complete response/complete response with incomplete count recovery of 75% and 57% in CRLF2r and non-CRLF2, respectively.14,18
Inotuzumab ozogamicin, a CD22 antibody drug-conjugate, has demonstrated a compositive complete response of 54% among 12 patients with Ph-like ALL and has yielded comparable response rates for Ph-like and non-Ph-like cohorts. The ongoing frontline studies evaluating the addition of blinatumomab (ECON-ACRIN E1910; NCT02003222)21 or inotuzumab ozogamicin (A041501 Alliance study; NCT03150693)22 to chemotherapy regimens in adults with newly diagnosed Ph-negative ALL could provide insights on its potential in Ph-like ALL.18
CD19-targeted chimeric antigen receptors have demonstrated excellent response rates in both adults and children with advanced B-ALL. Although no study has investigated these novel therapies specifically in Ph-like ALL, it is likely that a significant proportion of the patients have Ph-like disease.18 Table 2 shows the current targeted therapies under investigation for Ph-like ALL.
Table 2. Key cellular targeted therapies for Ph-like ALL*
| Therapy | Key cellular targets | Mechanism of action | Phase of clinical trials |
| Birinapant | TNF-α dependent | SMAC mimetic | In vitro and in vivo studies |
| CHZ868 | JAK2 mutated | Type 2 JAK2 inhibitor | In vitro and in vivo studies |
| Dasatinib | SRC/ABL class tyrosine kinase fusions | Type 2 SRC/ABL class tyrosine kinase inhibitor | Phase II and III clinical trials |
| Gedatolisib | PI3K and mTOR | Dual inhibitor of PI3K-α, PI3K-у, and mTOR |
In vitro and in vivo studies |
| Givinostat | CRLF2+ | Class 1 and 2 HDAC inhibitor | In vitro and in vivo studies |
| JQI | CRLF2+ | BET inhibitor | In vitro and in vivo studies |
| Ponatinib | SRC/ABL class tyrosine kinase fusions | Type 3 SRC/ABL class tyrosine kinase inhibitor | Single case study |
| Ruxolitinib | JAK2-mutated | Type I JAK2 inhibitor | Phase II clinical trials |
| Rapamycin | mTOR activated pathways | mTOR inhibitor | In vitro and in vivo studies |
| Luminespib | CRLF2+ | HSP90 inhibitor | In vitro and in vivo studies |
| Selumetinib and AZD1480 | CRLF2+ | MEK 1/2 inhibitor and ATP-competitive JAK2 inhibitor | In vitro studies |
| TSLPR CAR T cells | CRLF2+ | Allogeneic TSLPR CAR T cells | In vitro and in vivo studies |
|
CAR, chimeric antigen receptor; TSLPR, thymic stromal lymphopoietin receptor. |
|||
A summary of clinical trials on patients with newly diagnosed and R/R Ph-like ALL is shown in Table 3.
| NCT (status) | Treatment | Patient population | Phase and group (number of patients) |
| NCT028830499 (ongoing) |
Dasatinib | Newly diagnosed high-risk B-ALL including Ph-like ALL Age: 1−30 years |
Phase III COG trial (N = 5,956) |
| NCT027239946 (ongoing) |
Ruxolitinib, chemotherapy | Children with de-novo high-risk CRLF2-rearranged and/or JAK pathway-mutant ALL Age: 1−21 years |
Phase II COG trial (N = 170) |
| NCT031177517 (ongoing) |
Ruxolitinib, blinatumomab | Newly diagnosed patients with B-ALL Age: 1−18 years |
Phase II/III SJCRH trial (N = 1,000) |
| NCT035713218 (ongoing) |
Ruxolitinib | Newly diagnosed Ph-like ALL Age: 18−39.99 years |
Phase I; University of Chicago (N = 15) |
| NCT0364327623 (ongoing) |
Bortezomib, Blinatumomab, |
Newly diagnosed ALL Age: ≤17 years |
Phase III; AIEOP/BFM (N = 5,000) |
| NCT0300714711 (ongoing) |
Imatinib | Newly diagnosed ALL with ABL class fusion Age:2−21 years |
Phase III; COG/EsPhALL (N = 475) |
| NCT0450161424 (active; not recruiting) |
Ponatinib | Resistant/refractory Ph + or Ph-like ALL Age: ≥1−21 years |
Phase I/II; COG (N = 68) |
| NCT0383496113 (active; not recruiting) |
Larotrectinib | Relapsed acute leukemia with TRK fusion Age: 0-≤30 years |
Phase II; COG (N = 70) |
| NCT03040030 | Dasatinib | De novo B-All with ABL class fusion Age: ≥1−21 years |
Phase III; DFCI (N = 560) |
| NCT0391112825 (ongoing) |
Imatinib | Newly diagnosed ALL Age: ≥1–45 years |
Phase III; ALLtogether (N = 500) |
| NCT0214341410 | Dasatinib | Newly diagnosed or relapsed Ph-like ALL Age: ≥65 years |
Phase II; SWOG (N = 57) |
| NCT0327549326 (ongoing) NCT0361485827 |
CART-19/22 | Relapsed/refractory ALL Age: 6−65 years |
Phase I/II; Hospital of Soochow University (N = 17) |
| NCT0318112628 (completed) |
Venetoclax, navitoclax | Relapsed/refractory ALL Age: ≥4 years |
Phase I; Pullarkat, et al. (N = 69) |
| AIEOP, Associazione Italiana di Ematologia Oncologia Pediatrica; ALL, acute lymphoblastic leukemia; BFM, Berlin-Frankfurt-MünsteR; CAR-T, chimeric antigen receptor T-cell; COG, children’s oncology group; DFCI, Dana-Farber Cancer Institute; Ph, Philadelphia chromosome; SJCRH, St Jude Children’s Research Hospital; SWOG, Southwest Oncology Group. *Data from Alghandour, et al.29 |
|||
Key Guidelines and organizations
- ESMO clinical practice guidelines
- NCCN guidelines for physicians
- Cancer Research UK: treatment of acute lymphocytic leukemia.
- American Cancer Society: treatment of acute lymphocytic leukemia.
- Leukemia & Lymphoma Society: treatment of acute lymphocytic leukemia.
- NHS: treatment for acute lymphoblastic leukemia.
- NCCN guidelines for patients
- Patient empowerment network
- CancerCare
- Leukemia research foundation
- Know ALL
References
Please indicate your level of agreement with the following statements:
The content was clear and easy to understand
The content addressed the learning objectives
The content was relevant to my practice
I will change my clinical practice as a result of this content



